Deck 16: Acids and Bases
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/49
العب
ملء الشاشة (f)
Deck 16: Acids and Bases
1
A solution with a pH of 2.17 is
A) basic
B) acidic
C) neutral
A) basic
B) acidic
C) neutral
acidic
2
Which of the following is a conjugate acid-base pair?
A) HNO3-, H2NO3
B) HNH4, NH4+
C) H2F, HF-
D) HPO42-, PO43-
E) H2CN, CN-
A) HNO3-, H2NO3
B) HNH4, NH4+
C) H2F, HF-
D) HPO42-, PO43-
E) H2CN, CN-
HPO42-, PO43-
3
Which of the following is the strongest conjugate acid?
A) HF
B)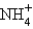
C) HOCl
D) HCN
E) all the same
A) HF
B)
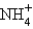
C) HOCl
D) HCN
E) all the same
HF
4
A solution where [H+] = 10-13 M is ______________.
A) basic
B) neutral
C) acidic
D) strongly acidic
E) two of these
A) basic
B) neutral
C) acidic
D) strongly acidic
E) two of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 49 في هذه المجموعة.
فتح الحزمة
k this deck
5
Which of the following is not a conjugate acid-base pair?
A) H2SO4, SO42-
B) HNO3, NO3-
C) HC2H3O2, C2H3O2-
D) H2PO4-, HPO42-
E) HBr, Br-
A) H2SO4, SO42-
B) HNO3, NO3-
C) HC2H3O2, C2H3O2-
D) H2PO4-, HPO42-
E) HBr, Br-

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 49 في هذه المجموعة.
فتح الحزمة
k this deck
6
Consider the reaction HC2H3O2(aq) + H2O(l) H3O+(aq) + C2H3O2-(aq). Which species is the conjugate acid?
A) HC2H3O2(aq)
B) H2O(l)
C) H3O+(aq)
D)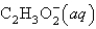
E) two of these
A) HC2H3O2(aq)
B) H2O(l)
C) H3O+(aq)
D)
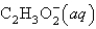
E) two of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 49 في هذه المجموعة.
فتح الحزمة
k this deck
7
Which of the following statements is/are correct?
A) In an acidic solution, [H+] > [OH-].
B) In a basic solution, [OH-] > [H+].
C) In a neutral solution, [H+] = [OH-].
D) None of the above statements (a-c) are correct.
E) All of the above statements (a-c) are correct.
A) In an acidic solution, [H+] > [OH-].
B) In a basic solution, [OH-] > [H+].
C) In a neutral solution, [H+] = [OH-].
D) None of the above statements (a-c) are correct.
E) All of the above statements (a-c) are correct.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 49 في هذه المجموعة.
فتح الحزمة
k this deck
8
Identify the Bronsted acids and bases in the following equation (A = Bronsted acid, B = Bronsted base): HSO3- + CN- HCN + SO32-
A) B A B A
B) B B A A
C) A B A B
D) A B B A
E) B A A B
A) B A B A
B) B B A A
C) A B A B
D) A B B A
E) B A A B

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 49 في هذه المجموعة.
فتح الحزمة
k this deck
9
A solution has [H+] = 4.0 *10-3 M. The [OH-] in this solution is
A)![<strong>A solution has [H<sup>+</sup>] = 4.0 *10<sup>-</sup><sup>3</sup> M. The [OH<sup>-</sup>] in this solution is</strong> A) M B) M C) M D) M E) none of these](https://d2lvgg3v3hfg70.cloudfront.net/TB6421/11eab077_f807_d161_beed_83914520f5b0_TB6421_11.jpg) M
M
B)![<strong>A solution has [H<sup>+</sup>] = 4.0 *10<sup>-</sup><sup>3</sup> M. The [OH<sup>-</sup>] in this solution is</strong> A) M B) M C) M D) M E) none of these](https://d2lvgg3v3hfg70.cloudfront.net/TB6421/11eab077_f807_d162_beed_43d73a80b722_TB6421_11.jpg) M
M
C)![<strong>A solution has [H<sup>+</sup>] = 4.0 *10<sup>-</sup><sup>3</sup> M. The [OH<sup>-</sup>] in this solution is</strong> A) M B) M C) M D) M E) none of these](https://d2lvgg3v3hfg70.cloudfront.net/TB6421/11eab077_f807_f873_beed_631c57563921_TB6421_11.jpg) M
M
D)![<strong>A solution has [H<sup>+</sup>] = 4.0 *10<sup>-</sup><sup>3</sup> M. The [OH<sup>-</sup>] in this solution is</strong> A) M B) M C) M D) M E) none of these](https://d2lvgg3v3hfg70.cloudfront.net/TB6421/11eab077_f807_f874_beed_ab35094464a0_TB6421_11.jpg) M
M
E) none of these
A)
![<strong>A solution has [H<sup>+</sup>] = 4.0 *10<sup>-</sup><sup>3</sup> M. The [OH<sup>-</sup>] in this solution is</strong> A) M B) M C) M D) M E) none of these](https://d2lvgg3v3hfg70.cloudfront.net/TB6421/11eab077_f807_d161_beed_83914520f5b0_TB6421_11.jpg) M
MB)
![<strong>A solution has [H<sup>+</sup>] = 4.0 *10<sup>-</sup><sup>3</sup> M. The [OH<sup>-</sup>] in this solution is</strong> A) M B) M C) M D) M E) none of these](https://d2lvgg3v3hfg70.cloudfront.net/TB6421/11eab077_f807_d162_beed_43d73a80b722_TB6421_11.jpg) M
MC)
![<strong>A solution has [H<sup>+</sup>] = 4.0 *10<sup>-</sup><sup>3</sup> M. The [OH<sup>-</sup>] in this solution is</strong> A) M B) M C) M D) M E) none of these](https://d2lvgg3v3hfg70.cloudfront.net/TB6421/11eab077_f807_f873_beed_631c57563921_TB6421_11.jpg) M
MD)
![<strong>A solution has [H<sup>+</sup>] = 4.0 *10<sup>-</sup><sup>3</sup> M. The [OH<sup>-</sup>] in this solution is</strong> A) M B) M C) M D) M E) none of these](https://d2lvgg3v3hfg70.cloudfront.net/TB6421/11eab077_f807_f874_beed_ab35094464a0_TB6421_11.jpg) M
ME) none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 49 في هذه المجموعة.
فتح الحزمة
k this deck
10
A solution has [OH-] = 3.0 *10-7 M. The [H+] in this solution is
A) 1.0 M
B)![<strong>A solution has [OH<sup>-</sup>] = 3.0 *10<sup>-</sup><sup>7</sup> M. The [H<sup>+</sup>] in this solution is</strong> A) 1.0 M B) M C) M D) M E) none of these](https://d2lvgg3v3hfg70.cloudfront.net/TB6421/11eab077_f808_1f85_beed_3740742d5441_TB6421_11.jpg) M
M
C)![<strong>A solution has [OH<sup>-</sup>] = 3.0 *10<sup>-</sup><sup>7</sup> M. The [H<sup>+</sup>] in this solution is</strong> A) 1.0 M B) M C) M D) M E) none of these](https://d2lvgg3v3hfg70.cloudfront.net/TB6421/11eab077_f808_1f86_beed_1dde101606ae_TB6421_11.jpg) M
M
D)![<strong>A solution has [OH<sup>-</sup>] = 3.0 *10<sup>-</sup><sup>7</sup> M. The [H<sup>+</sup>] in this solution is</strong> A) 1.0 M B) M C) M D) M E) none of these](https://d2lvgg3v3hfg70.cloudfront.net/TB6421/11eab077_f808_4697_beed_3f3528fdf2a5_TB6421_11.jpg) M
M
E) none of these
A) 1.0 M
B)
![<strong>A solution has [OH<sup>-</sup>] = 3.0 *10<sup>-</sup><sup>7</sup> M. The [H<sup>+</sup>] in this solution is</strong> A) 1.0 M B) M C) M D) M E) none of these](https://d2lvgg3v3hfg70.cloudfront.net/TB6421/11eab077_f808_1f85_beed_3740742d5441_TB6421_11.jpg) M
MC)
![<strong>A solution has [OH<sup>-</sup>] = 3.0 *10<sup>-</sup><sup>7</sup> M. The [H<sup>+</sup>] in this solution is</strong> A) 1.0 M B) M C) M D) M E) none of these](https://d2lvgg3v3hfg70.cloudfront.net/TB6421/11eab077_f808_1f86_beed_1dde101606ae_TB6421_11.jpg) M
MD)
![<strong>A solution has [OH<sup>-</sup>] = 3.0 *10<sup>-</sup><sup>7</sup> M. The [H<sup>+</sup>] in this solution is</strong> A) 1.0 M B) M C) M D) M E) none of these](https://d2lvgg3v3hfg70.cloudfront.net/TB6421/11eab077_f808_4697_beed_3f3528fdf2a5_TB6421_11.jpg) M
ME) none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 49 في هذه المجموعة.
فتح الحزمة
k this deck
11
Calculate the [H+] in a solution that has a pH of 8.82.
A)![<strong>Calculate the [H<sup>+</sup>] in a solution that has a pH of 8.82.</strong> A) M B) M C) M D) M E) none of these](https://d2lvgg3v3hfg70.cloudfront.net/TB6421/11eab077_f808_4698_beed_256d2eabfe3b_TB6421_11.jpg) M
M
B)![<strong>Calculate the [H<sup>+</sup>] in a solution that has a pH of 8.82.</strong> A) M B) M C) M D) M E) none of these](https://d2lvgg3v3hfg70.cloudfront.net/TB6421/11eab077_f808_6da9_beed_c1e17ba8fb4f_TB6421_00.jpg) M
M
C)![<strong>Calculate the [H<sup>+</sup>] in a solution that has a pH of 8.82.</strong> A) M B) M C) M D) M E) none of these](https://d2lvgg3v3hfg70.cloudfront.net/TB6421/11eab077_f808_6daa_beed_dd6871eca352_TB6421_11.jpg) M
M
D)![<strong>Calculate the [H<sup>+</sup>] in a solution that has a pH of 8.82.</strong> A) M B) M C) M D) M E) none of these](https://d2lvgg3v3hfg70.cloudfront.net/TB6421/11eab077_f808_94bb_beed_3b23de5e15aa_TB6421_11.jpg) M
M
E) none of these
A)
![<strong>Calculate the [H<sup>+</sup>] in a solution that has a pH of 8.82.</strong> A) M B) M C) M D) M E) none of these](https://d2lvgg3v3hfg70.cloudfront.net/TB6421/11eab077_f808_4698_beed_256d2eabfe3b_TB6421_11.jpg) M
MB)
![<strong>Calculate the [H<sup>+</sup>] in a solution that has a pH of 8.82.</strong> A) M B) M C) M D) M E) none of these](https://d2lvgg3v3hfg70.cloudfront.net/TB6421/11eab077_f808_6da9_beed_c1e17ba8fb4f_TB6421_00.jpg) M
MC)
![<strong>Calculate the [H<sup>+</sup>] in a solution that has a pH of 8.82.</strong> A) M B) M C) M D) M E) none of these](https://d2lvgg3v3hfg70.cloudfront.net/TB6421/11eab077_f808_6daa_beed_dd6871eca352_TB6421_11.jpg) M
MD)
![<strong>Calculate the [H<sup>+</sup>] in a solution that has a pH of 8.82.</strong> A) M B) M C) M D) M E) none of these](https://d2lvgg3v3hfg70.cloudfront.net/TB6421/11eab077_f808_94bb_beed_3b23de5e15aa_TB6421_11.jpg) M
ME) none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 49 في هذه المجموعة.
فتح الحزمة
k this deck
12
The conjugate acid of a weak base is a strong acid.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 49 في هذه المجموعة.
فتح الحزمة
k this deck
13
A strong acid is one for which the forward reaction predominates.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 49 في هذه المجموعة.
فتح الحزمة
k this deck
14
Arrhenius postulated that acids produce hydrogen ions in aqueous solution, whereas bases produce hydroxide ions.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 49 في هذه المجموعة.
فتح الحزمة
k this deck
15
Choose the case that is not a Bronsted conjugate acid-base pair.
A) CH3NH3+, CH3NH2
B) HCN, CN-
C) HClO2, ClO2-
D) HCO2H, HCOH
E) H3BO3, H2BO3-
A) CH3NH3+, CH3NH2
B) HCN, CN-
C) HClO2, ClO2-
D) HCO2H, HCOH
E) H3BO3, H2BO3-

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 49 في هذه المجموعة.
فتح الحزمة
k this deck
16
Choose the pair of concentrations that cannot be in a given aqueous solution at 25°C.
A) [H+] = 10-3 M, [OH-] = 10-11 M
B) [H+] = 10-7 M, [OH-] = 10-7 M
C) [H+] = 10-13 M, [OH-] = 1 M
D) [H+] = 10 M, [OH-] = 10-15 M
E) All of these can exist.
A) [H+] = 10-3 M, [OH-] = 10-11 M
B) [H+] = 10-7 M, [OH-] = 10-7 M
C) [H+] = 10-13 M, [OH-] = 1 M
D) [H+] = 10 M, [OH-] = 10-15 M
E) All of these can exist.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 49 في هذه المجموعة.
فتح الحزمة
k this deck
17
A solution is buffered by the presence of a weak acid and its conjugate base.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 49 في هذه المجموعة.
فتح الحزمة
k this deck
18
Which of the following is the strongest conjugate base?
A) F-
B) NH3
C) OCl-
D) CN-
E) all the same
A) F-
B) NH3
C) OCl-
D) CN-
E) all the same

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 49 في هذه المجموعة.
فتح الحزمة
k this deck
19
Which has the higher [H+], 0.40 M RbOH or 0.20 M Ca(OH)2?
A) Neither, the [H+]'s are equal.
B) 0.40 M RbOH
C) 0.20 M Ca(OH)2
D) Neither, these are bases so there is no [H+].
E) The answer depends on the volumes of the respective solutions.
A) Neither, the [H+]'s are equal.
B) 0.40 M RbOH
C) 0.20 M Ca(OH)2
D) Neither, these are bases so there is no [H+].
E) The answer depends on the volumes of the respective solutions.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 49 في هذه المجموعة.
فتح الحزمة
k this deck
20
As water is heated, its [H+] increases. This means that
A) the water is no longer neutral
B) [H+] > [OH-]
C) [OH-] > [H+]
D) a and b are correct
E) none of these
A) the water is no longer neutral
B) [H+] > [OH-]
C) [OH-] > [H+]
D) a and b are correct
E) none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 49 في هذه المجموعة.
فتح الحزمة
k this deck
21
What is the pH of a solution prepared by dissolving 96.1 g NaOH in enough water to make 3.2 L of solution?
A) 13.88
B) 0.12
C) 14.12
D) 10.28
E) none of these
A) 13.88
B) 0.12
C) 14.12
D) 10.28
E) none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 49 في هذه المجموعة.
فتح الحزمة
k this deck
22
The pH of a solution at 25oC in which [OH-] = 3.5 *10-5 M is
A) 4.46
B) 4.77
C) 9.54
D) 11.04
E) none of these
A) 4.46
B) 4.77
C) 9.54
D) 11.04
E) none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 49 في هذه المجموعة.
فتح الحزمة
k this deck
23
How many moles of pure NaOH must be used to prepare 1.0 L of a solution that has pH = 12.26?
A) 8.2 * 10-16 mol
B) 0.018 mol
C) 5.5 * 10-13 mol
D) 1.74 mol
E) none of these
A) 8.2 * 10-16 mol
B) 0.018 mol
C) 5.5 * 10-13 mol
D) 1.74 mol
E) none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 49 في هذه المجموعة.
فتح الحزمة
k this deck
24
Calculate the [H+] in a solution that has a pH of 5.15.
A)![<strong>Calculate the [H<sup>+</sup>] in a solution that has a pH of 5.15.</strong> A) M B) M C) 8.85 M D) 5.15 M E) none of these](https://d2lvgg3v3hfg70.cloudfront.net/TB6421/11eab077_f809_3102_beed_15f8c0790253_TB6421_11.jpg) M
M
B)![<strong>Calculate the [H<sup>+</sup>] in a solution that has a pH of 5.15.</strong> A) M B) M C) 8.85 M D) 5.15 M E) none of these](https://d2lvgg3v3hfg70.cloudfront.net/TB6421/11eab077_f809_3103_beed_0fe2cd30f259_TB6421_11.jpg) M
M
C) 8.85 M
D) 5.15 M
E) none of these
A)
![<strong>Calculate the [H<sup>+</sup>] in a solution that has a pH of 5.15.</strong> A) M B) M C) 8.85 M D) 5.15 M E) none of these](https://d2lvgg3v3hfg70.cloudfront.net/TB6421/11eab077_f809_3102_beed_15f8c0790253_TB6421_11.jpg) M
MB)
![<strong>Calculate the [H<sup>+</sup>] in a solution that has a pH of 5.15.</strong> A) M B) M C) 8.85 M D) 5.15 M E) none of these](https://d2lvgg3v3hfg70.cloudfront.net/TB6421/11eab077_f809_3103_beed_0fe2cd30f259_TB6421_11.jpg) M
MC) 8.85 M
D) 5.15 M
E) none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 49 في هذه المجموعة.
فتح الحزمة
k this deck
25
Calculate the [OH-] in a solution that has a pH of 3.76.
A)![<strong>Calculate the [OH<sup>-</sup>] in a solution that has a pH of 3.76.</strong> A) M B) M C) M D) M E) none of these](https://d2lvgg3v3hfg70.cloudfront.net/TB6421/11eab077_f808_94bc_beed_8351ed7a6a14_TB6421_11.jpg) M
M
B)![<strong>Calculate the [OH<sup>-</sup>] in a solution that has a pH of 3.76.</strong> A) M B) M C) M D) M E) none of these](https://d2lvgg3v3hfg70.cloudfront.net/TB6421/11eab077_f808_bbcd_beed_3d472243c1ba_TB6421_11.jpg) M
M
C)![<strong>Calculate the [OH<sup>-</sup>] in a solution that has a pH of 3.76.</strong> A) M B) M C) M D) M E) none of these](https://d2lvgg3v3hfg70.cloudfront.net/TB6421/11eab077_f808_bbce_beed_63a7136d55f9_TB6421_11.jpg) M
M
D)![<strong>Calculate the [OH<sup>-</sup>] in a solution that has a pH of 3.76.</strong> A) M B) M C) M D) M E) none of these](https://d2lvgg3v3hfg70.cloudfront.net/TB6421/11eab077_f808_e2df_beed_85bf5aa8fad5_TB6421_11.jpg) M
M
E) none of these
A)
![<strong>Calculate the [OH<sup>-</sup>] in a solution that has a pH of 3.76.</strong> A) M B) M C) M D) M E) none of these](https://d2lvgg3v3hfg70.cloudfront.net/TB6421/11eab077_f808_94bc_beed_8351ed7a6a14_TB6421_11.jpg) M
MB)
![<strong>Calculate the [OH<sup>-</sup>] in a solution that has a pH of 3.76.</strong> A) M B) M C) M D) M E) none of these](https://d2lvgg3v3hfg70.cloudfront.net/TB6421/11eab077_f808_bbcd_beed_3d472243c1ba_TB6421_11.jpg) M
MC)
![<strong>Calculate the [OH<sup>-</sup>] in a solution that has a pH of 3.76.</strong> A) M B) M C) M D) M E) none of these](https://d2lvgg3v3hfg70.cloudfront.net/TB6421/11eab077_f808_bbce_beed_63a7136d55f9_TB6421_11.jpg) M
MD)
![<strong>Calculate the [OH<sup>-</sup>] in a solution that has a pH of 3.76.</strong> A) M B) M C) M D) M E) none of these](https://d2lvgg3v3hfg70.cloudfront.net/TB6421/11eab077_f808_e2df_beed_85bf5aa8fad5_TB6421_11.jpg) M
ME) none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 49 في هذه المجموعة.
فتح الحزمة
k this deck
26
A solution has [H+] = 5.0 *10-8 M. The pH of this solution is
A) 6.70
B) 5.98
C) 7.30
D) 9.77
E) none of these
A) 6.70
B) 5.98
C) 7.30
D) 9.77
E) none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 49 في هذه المجموعة.
فتح الحزمة
k this deck
27
A solution has [H+] = 4.3 *10-8 M. The pOH of this solution is
A) 7.37
B) 6.63
C) 9.37
D) 3.28
E) none of these
A) 7.37
B) 6.63
C) 9.37
D) 3.28
E) none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 49 في هذه المجموعة.
فتح الحزمة
k this deck
28
A solution is prepared by dissolving 111.9 g HCl(g) in enough water to make 160.0 L of solution. The pH of this solution is
A) 1.72
B) 12.28
C) 0.155
D) 3.07
E) none of these
A) 1.72
B) 12.28
C) 0.155
D) 3.07
E) none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 49 في هذه المجموعة.
فتح الحزمة
k this deck
29
A solution has [OH-]= 5.0 *10-4 M. The pH of this solution is
A) 3.30
B) 2.00 * 10-11
C) 5.35
D) 10.70
E) none of these
A) 3.30
B) 2.00 * 10-11
C) 5.35
D) 10.70
E) none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 49 في هذه المجموعة.
فتح الحزمة
k this deck
30
What is the pH of a solution that has [OH-] = ![<strong>What is the pH of a solution that has [OH<sup>-</sup>] = .</strong> A) 4.93 M B) 9.07 M C) 2.46 M D) 7.14 M E) none of these](https://d2lvgg3v3hfg70.cloudfront.net/TB6421/11eab077_f80a_1b69_beed_2d9c0f2ede1c_TB6421_11.jpg) .
.
A) 4.93 M
B) 9.07 M
C) 2.46 M
D) 7.14 M
E) none of these
![<strong>What is the pH of a solution that has [OH<sup>-</sup>] = .</strong> A) 4.93 M B) 9.07 M C) 2.46 M D) 7.14 M E) none of these](https://d2lvgg3v3hfg70.cloudfront.net/TB6421/11eab077_f80a_1b69_beed_2d9c0f2ede1c_TB6421_11.jpg) .
.A) 4.93 M
B) 9.07 M
C) 2.46 M
D) 7.14 M
E) none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 49 في هذه المجموعة.
فتح الحزمة
k this deck
31
Which statement is true for a strong base solution with a concentration greater than 1.0 M?
A) pOH > pH
B) pH > pOH
C) pH < 0
D) pH<14
E) Two of these (a-d) are true.
A) pOH > pH
B) pH > pOH
C) pH < 0
D) pH<14
E) Two of these (a-d) are true.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 49 في هذه المجموعة.
فتح الحزمة
k this deck
32
Calculate the [H+] in a solution that has a pH of 9.16.
A)![<strong>Calculate the [H<sup>+</sup>] in a solution that has a pH of 9.16.</strong> A) M B) M C) 4.84 M D) 9.16 M E) none of these](https://d2lvgg3v3hfg70.cloudfront.net/TB6421/11eab077_f809_09f0_beed_11cd8bf6f992_TB6421_11.jpg) M
M
B)![<strong>Calculate the [H<sup>+</sup>] in a solution that has a pH of 9.16.</strong> A) M B) M C) 4.84 M D) 9.16 M E) none of these](https://d2lvgg3v3hfg70.cloudfront.net/TB6421/11eab077_f809_09f1_beed_7b959b2c4cba_TB6421_11.jpg) M
M
C) 4.84 M
D) 9.16 M
E) none of these
A)
![<strong>Calculate the [H<sup>+</sup>] in a solution that has a pH of 9.16.</strong> A) M B) M C) 4.84 M D) 9.16 M E) none of these](https://d2lvgg3v3hfg70.cloudfront.net/TB6421/11eab077_f809_09f0_beed_11cd8bf6f992_TB6421_11.jpg) M
MB)
![<strong>Calculate the [H<sup>+</sup>] in a solution that has a pH of 9.16.</strong> A) M B) M C) 4.84 M D) 9.16 M E) none of these](https://d2lvgg3v3hfg70.cloudfront.net/TB6421/11eab077_f809_09f1_beed_7b959b2c4cba_TB6421_11.jpg) M
MC) 4.84 M
D) 9.16 M
E) none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 49 في هذه المجموعة.
فتح الحزمة
k this deck
33
Calculate the [H+] in a solution that has a pH of 5.54.
A) 8.46
B)![<strong>Calculate the [H<sup>+</sup>] in a solution that has a pH of 5.54.</strong> A) 8.46 B) M C) 5.54 D) M E) none of these](https://d2lvgg3v3hfg70.cloudfront.net/TB6421/11eab077_f809_cd47_beed_bdeb537d17bf_TB6421_11.jpg) M
M
C) 5.54
D)![<strong>Calculate the [H<sup>+</sup>] in a solution that has a pH of 5.54.</strong> A) 8.46 B) M C) 5.54 D) M E) none of these](https://d2lvgg3v3hfg70.cloudfront.net/TB6421/11eab077_f809_cd48_beed_bdf41ea7140c_TB6421_11.jpg) M
M
E) none of these
A) 8.46
B)
![<strong>Calculate the [H<sup>+</sup>] in a solution that has a pH of 5.54.</strong> A) 8.46 B) M C) 5.54 D) M E) none of these](https://d2lvgg3v3hfg70.cloudfront.net/TB6421/11eab077_f809_cd47_beed_bdeb537d17bf_TB6421_11.jpg) M
MC) 5.54
D)
![<strong>Calculate the [H<sup>+</sup>] in a solution that has a pH of 5.54.</strong> A) 8.46 B) M C) 5.54 D) M E) none of these](https://d2lvgg3v3hfg70.cloudfront.net/TB6421/11eab077_f809_cd48_beed_bdf41ea7140c_TB6421_11.jpg) M
ME) none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 49 في هذه المجموعة.
فتح الحزمة
k this deck
34
Solid calcium hydroxide is dissolved in water until the pH of the solution is 10.39. The hydroxide ion concentration [OH-] of the solution is
A)![<strong>Solid calcium hydroxide is dissolved in water until the pH of the solution is 10.39. The hydroxide ion concentration [OH<sup>-</sup>] of the solution is</strong> A) M B) 3.61 M C) M D) M E) none of these](https://d2lvgg3v3hfg70.cloudfront.net/TB6421/11eab077_f809_5814_beed_612f4019727e_TB6421_11.jpg) M
M
B) 3.61 M
C)![<strong>Solid calcium hydroxide is dissolved in water until the pH of the solution is 10.39. The hydroxide ion concentration [OH<sup>-</sup>] of the solution is</strong> A) M B) 3.61 M C) M D) M E) none of these](https://d2lvgg3v3hfg70.cloudfront.net/TB6421/11eab077_f809_7f25_beed_6369f87fddae_TB6421_11.jpg) M
M
D)![<strong>Solid calcium hydroxide is dissolved in water until the pH of the solution is 10.39. The hydroxide ion concentration [OH<sup>-</sup>] of the solution is</strong> A) M B) 3.61 M C) M D) M E) none of these](https://d2lvgg3v3hfg70.cloudfront.net/TB6421/11eab077_f809_7f26_beed_458ed36be8c8_TB6421_11.jpg) M
M
E) none of these
A)
![<strong>Solid calcium hydroxide is dissolved in water until the pH of the solution is 10.39. The hydroxide ion concentration [OH<sup>-</sup>] of the solution is</strong> A) M B) 3.61 M C) M D) M E) none of these](https://d2lvgg3v3hfg70.cloudfront.net/TB6421/11eab077_f809_5814_beed_612f4019727e_TB6421_11.jpg) M
MB) 3.61 M
C)
![<strong>Solid calcium hydroxide is dissolved in water until the pH of the solution is 10.39. The hydroxide ion concentration [OH<sup>-</sup>] of the solution is</strong> A) M B) 3.61 M C) M D) M E) none of these](https://d2lvgg3v3hfg70.cloudfront.net/TB6421/11eab077_f809_7f25_beed_6369f87fddae_TB6421_11.jpg) M
MD)
![<strong>Solid calcium hydroxide is dissolved in water until the pH of the solution is 10.39. The hydroxide ion concentration [OH<sup>-</sup>] of the solution is</strong> A) M B) 3.61 M C) M D) M E) none of these](https://d2lvgg3v3hfg70.cloudfront.net/TB6421/11eab077_f809_7f26_beed_458ed36be8c8_TB6421_11.jpg) M
ME) none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 49 في هذه المجموعة.
فتح الحزمة
k this deck
35
A solution has a pH of 4.42. The [H+] in this solution is
A) 2.6 * 10-10 M
B) 3.8 * 10-5 M
C) 9.58
D) 4.42
E) none of these
A) 2.6 * 10-10 M
B) 3.8 * 10-5 M
C) 9.58
D) 4.42
E) none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 49 في هذه المجموعة.
فتح الحزمة
k this deck
36
A solution with a pH of 2 is how many times more acidic as a solution with a pH of 6?
A) 3
B) 0.3
C) 100000
D) 10000
E) 8
A) 3
B) 0.3
C) 100000
D) 10000
E) 8

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 49 في هذه المجموعة.
فتح الحزمة
k this deck
37
A solution has a pH of 3.16. The pOH of this solution is
A) 3.26
B) 10.74
C) 3.16
D) 10.84
E) none of these
A) 3.26
B) 10.74
C) 3.16
D) 10.84
E) none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 49 في هذه المجموعة.
فتح الحزمة
k this deck
38
A solution with a pH of 3 is how many times as acidic as a solution with a pH of 5? (Ignore significant figures for this problem.)
A) 0.6 times as acidic
B) 2 times as acidic
C) 100 times as acidic
D) 15 times as acidic
E) 8 times as acidic
A) 0.6 times as acidic
B) 2 times as acidic
C) 100 times as acidic
D) 15 times as acidic
E) 8 times as acidic

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 49 في هذه المجموعة.
فتح الحزمة
k this deck
39
Calculate the pH of a 0.045 M HCl solution.
A) 1.23
B) 1.35
C) 12.65
D) 12.29
E) none of these
A) 1.23
B) 1.35
C) 12.65
D) 12.29
E) none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 49 في هذه المجموعة.
فتح الحزمة
k this deck
40
Calculate the pH of an acid solution containing 0.044 M HNO3.
A) 12.64
B) 2.81
C) 1.36
D) 0.044
E) none of these
A) 12.64
B) 2.81
C) 1.36
D) 0.044
E) none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 49 في هذه المجموعة.
فتح الحزمة
k this deck
41
Calculate the pH of 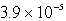 M HCl.
M HCl.
A) 4.41
B) 9.59
C) 13.75
D) 6.90
E) none of these
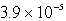 M HCl.
M HCl.A) 4.41
B) 9.59
C) 13.75
D) 6.90
E) none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 49 في هذه المجموعة.
فتح الحزمة
k this deck
42
Calculate the pH of 0.056 M HClO4.
A) 0.056
B) 7.00
C) 1.25
D) 12.75
E) none of these
A) 0.056
B) 7.00
C) 1.25
D) 12.75
E) none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 49 في هذه المجموعة.
فتح الحزمة
k this deck
43
A weak acid, HOCl, is in solution with dissolved sodium hypochlorite, NaOCl. If HCl is added which ion will react with the extra hydrogen ions from the HCl to keep the pH from changing?
A) OCl-
B) Na+
C) HOCl-
D) OH-
E) none of these
A) OCl-
B) Na+
C) HOCl-
D) OH-
E) none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 49 في هذه المجموعة.
فتح الحزمة
k this deck
44
A buffered solution contains HNO2. It also contains
A) KCl
B) HNO3
C) KOH
D) KNO2
E) NaCl
A) KCl
B) HNO3
C) KOH
D) KNO2
E) NaCl

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 49 في هذه المجموعة.
فتح الحزمة
k this deck
45
What is the pH of a 4.9 M solution of HClO4?
A) 14.69
B) 0.69
C) -0.69
D) 13.31
E) minus infinity
A) 14.69
B) 0.69
C) -0.69
D) 13.31
E) minus infinity

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 49 في هذه المجموعة.
فتح الحزمة
k this deck
46
What is the pH of a 2.1 M solution of HNO3?
A) -0.32
B) 0.32
C) 14.32
D) 13.68
E) none of these
A) -0.32
B) 0.32
C) 14.32
D) 13.68
E) none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 49 في هذه المجموعة.
فتح الحزمة
k this deck
47
What is the pH of a 0.25 M nitric acid (HNO3) solution?
A) 0.60
B) 2.50
C) 2.49
D) 13.40
E) none of these
A) 0.60
B) 2.50
C) 2.49
D) 13.40
E) none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 49 في هذه المجموعة.
فتح الحزمة
k this deck
48
Which of the following is true for a buffered solution?
A) The solution resists any change in its [H+].
B) The solution will not change its pH very much even if a concentrated acid is added.
C) The solution will not change its pH very much even if a strong base is added.
D) Any H+ ions added will react with a conjugate base of a weak acid already in solution.
E) all of these
A) The solution resists any change in its [H+].
B) The solution will not change its pH very much even if a concentrated acid is added.
C) The solution will not change its pH very much even if a strong base is added.
D) Any H+ ions added will react with a conjugate base of a weak acid already in solution.
E) all of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 49 في هذه المجموعة.
فتح الحزمة
k this deck
49
A NaCl(aq) solution is automatically buffered.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 49 في هذه المجموعة.
فتح الحزمة
k this deck








