Deck 7: Reactions in Aqueous Solutions
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/64
العب
ملء الشاشة (f)
Deck 7: Reactions in Aqueous Solutions
1
Write and balance molecular equations for the following reactions between aqueous solutions. You will need to decide on the formulas and phases of the products in each of the cases.
-An aqueous solution of calcium nitrate is mixed with an aqueous solution of sodium phosphate.
-An aqueous solution of calcium nitrate is mixed with an aqueous solution of sodium phosphate.
3Ca(NO3)2(aq) + 2Na3PO4(aq) Ca3(PO4)2(s) + 6NaNO3(aq)
2
An aqueous solution of sodium sulfate is allowed to react with an aqueous solution of calcium nitrate. The complete ionic equation contains which of the following species (when balanced in standard form)?.
A) 2Na+(aq)
B) 2SO42-(aq)
C) 3Ca2+(aq)
D) NO3-(aq)
E) K+(aq)
A) 2Na+(aq)
B) 2SO42-(aq)
C) 3Ca2+(aq)
D) NO3-(aq)
E) K+(aq)
2Na+(aq)
3
Write and balance molecular equations for the following reactions between aqueous solutions. You will need to decide on the formulas and phases of the products in each of the cases.
-An aqueous solution of silver nitrate is mixed with an aqueous solution of potassium chromate.
-An aqueous solution of silver nitrate is mixed with an aqueous solution of potassium chromate.
2AgNO3(aq) + K2CrO4(aq) Ag2CrO4(s) + 2KNO3(aq)
4
A substance that, when dissolved in water, produces a solution that conducts electric current very efficiently is called
A) a strong electrolyte
B) a weak electrolyte
C) a strong ion
D) an electrical solute
E) none of these
A) a strong electrolyte
B) a weak electrolyte
C) a strong ion
D) an electrical solute
E) none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 64 في هذه المجموعة.
فتح الحزمة
k this deck
5
Write and balance molecular equations for the following reactions between aqueous solutions. You will need to decide on the formulas and phases of the products in each of the cases.
-An aqueous solution of lead(II) nitrate is mixed with an aqueous solution of potassium chloride.
-An aqueous solution of lead(II) nitrate is mixed with an aqueous solution of potassium chloride.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 64 في هذه المجموعة.
فتح الحزمة
k this deck
6
Write and balance molecular equations for the following reactions between aqueous solutions. You will need to decide on the formulas and phases of the products in each of the cases.
-An aqueous solution of lead(II) nitrate is mixed with an aqueous solution of sodium iodide.
-An aqueous solution of lead(II) nitrate is mixed with an aqueous solution of sodium iodide.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 64 في هذه المجموعة.
فتح الحزمة
k this deck
7
Write and balance molecular equations for the following reactions between aqueous solutions. You will need to decide on the formulas and phases of the products in each of the cases.
-An aqueous solution of potassium chloride is mixed with an aqueous solution of sodium sulfate.
-An aqueous solution of potassium chloride is mixed with an aqueous solution of sodium sulfate.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 64 في هذه المجموعة.
فتح الحزمة
k this deck
8
An aqueous solution of potassium sulfate is allowed to react with an aqueous solution of lead(II) nitrate. The net ionic equation contains which of the following species (when balanced in standard form)?.
A) Pb2+(aq)
B) 2SO42-(aq)
C) 2K+(aq)
D) NO3-(aq)
E) 2NO3-(aq)
A) Pb2+(aq)
B) 2SO42-(aq)
C) 2K+(aq)
D) NO3-(aq)
E) 2NO3-(aq)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 64 في هذه المجموعة.
فتح الحزمة
k this deck
9
Write the balanced molecular equation for the reaction between aqueous solutions of lithium phosphate and sodium hydroxide.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 64 في هذه المجموعة.
فتح الحزمة
k this deck
10
When a precipitation reaction occurs, the ions that do not form the precipitate
A) evaporate
B) are cations only
C) form a second insoluble compound in the solution
D) are left dissolved in the solution
E) none of these
A) evaporate
B) are cations only
C) form a second insoluble compound in the solution
D) are left dissolved in the solution
E) none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 64 في هذه المجموعة.
فتح الحزمة
k this deck
11
Write and balance molecular equations for the following reactions between aqueous solutions. You will need to decide on the formulas and phases of the products in each of the cases.
-An aqueous solution of copper(II) nitrate is mixed with an aqueous solution of sodium hydroxide.
-An aqueous solution of copper(II) nitrate is mixed with an aqueous solution of sodium hydroxide.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 64 في هذه المجموعة.
فتح الحزمة
k this deck
12
An aqueous solution of potassium sulfate is allowed to react with an aqueous solution of barium nitrate. What is the coefficient of the solid in the balanced equation (in standard form)?
A) 1
B) 2
C) 3
D) 4
E) 6
A) 1
B) 2
C) 3
D) 4
E) 6

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 64 في هذه المجموعة.
فتح الحزمة
k this deck
13
Which drawing best represents the mixing of aqueous calcium chloride with aqueous potassium sulfate when they are mixed in stoichiometric amounts (neither reactant is limiting)?
A)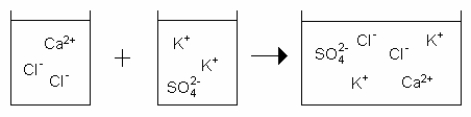
B)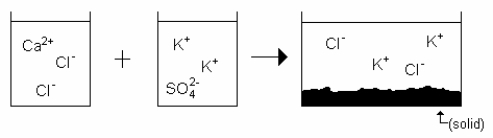
C)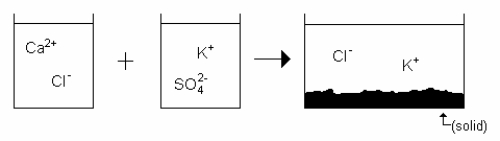
D)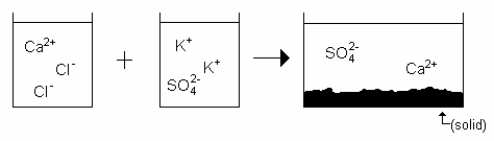
E)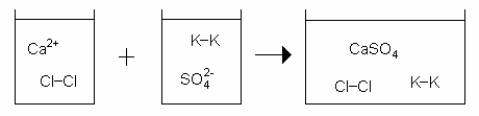
A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 64 في هذه المجموعة.
فتح الحزمة
k this deck
14
Write and balance molecular equations for the following reactions between aqueous solutions. You will need to decide on the formulas and phases of the products in each of the cases.
-An aqueous solution of barium hydroxide is mixed with an aqueous solution of sulfuric acid.
-An aqueous solution of barium hydroxide is mixed with an aqueous solution of sulfuric acid.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 64 في هذه المجموعة.
فتح الحزمة
k this deck
15
Write and balance molecular equations for the following reactions between aqueous solutions. You will need to decide on the formulas and phases of the products in each of the cases.
-An aqueous solution of barium nitrate is mixed with an aqueous solution of potassium phosphate.
-An aqueous solution of barium nitrate is mixed with an aqueous solution of potassium phosphate.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 64 في هذه المجموعة.
فتح الحزمة
k this deck
16
The factors that most commonly cause chemical reactions to occur are all the following except
A) formation of a solid
B) formation of a gas
C) formation of water
D) transfer of electrons
E) a decrease in temperature
A) formation of a solid
B) formation of a gas
C) formation of water
D) transfer of electrons
E) a decrease in temperature

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 64 في هذه المجموعة.
فتح الحزمة
k this deck
17
Complete and write the balanced molecular equation for the following: An aqueous solution of magnesium chloride is added to an aqueous solution of silver nitrate.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 64 في هذه المجموعة.
فتح الحزمة
k this deck
18
When solutions of barium hydroxide and sulfuric acid are mixed, the net ionic equation is
Ba2+(aq) + SO42-(aq) BaSO4(s) because only the species involved in making the precipitate are included.
Ba2+(aq) + SO42-(aq) BaSO4(s) because only the species involved in making the precipitate are included.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 64 في هذه المجموعة.
فتح الحزمة
k this deck
19
An aqueous solution of ammonium sulfate is allowed to react with an aqueous solution of barium nitrate. Identify the solid in the balanced equation.
A) BaSO4
B) (NH4)2SO4
C) Ba(NO3)2
D) NH4NO3
E) There is no solid formed when the two solutions are mixed.
A) BaSO4
B) (NH4)2SO4
C) Ba(NO3)2
D) NH4NO3
E) There is no solid formed when the two solutions are mixed.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 64 في هذه المجموعة.
فتح الحزمة
k this deck
20
Write and balance molecular equations for the following reactions between aqueous solutions. You will need to decide on the formulas and phases of the products in each of the cases.
-An aqueous solution of silver nitrate is mixed with an aqueous solution of potassium carbonate.
-An aqueous solution of silver nitrate is mixed with an aqueous solution of potassium carbonate.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 64 في هذه المجموعة.
فتح الحزمة
k this deck
21
Electrons are transferred in combustion reactions.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 64 في هذه المجموعة.
فتح الحزمة
k this deck
22
An aqueous solution of sodium sulfide is allowed to react with an aqueous solution of barium chloride. The complete ionic equation contains which of the following species (when balanced in standard form)?
A) 2Na+(aq)
B) Na+(aq)
C) 3Ba2+(aq)
D) 2S2-(aq)
E) Cl-(aq)
A) 2Na+(aq)
B) Na+(aq)
C) 3Ba2+(aq)
D) 2S2-(aq)
E) Cl-(aq)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 64 في هذه المجموعة.
فتح الحزمة
k this deck
23
An aqueous solution of sodium carbonate is allowed to react with an aqueous solution of magnesium chloride. The complete ionic equation contains which of the following species (when balanced in standard form)?
A) CO32-(aq)
B) 2Na+(aq)
C) 2Mg2+(aq)
D) Cl-(aq)
E) 2Cl-(aq)
A) CO32-(aq)
B) 2Na+(aq)
C) 2Mg2+(aq)
D) Cl-(aq)
E) 2Cl-(aq)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 64 في هذه المجموعة.
فتح الحزمة
k this deck
24
An aqueous solution of potassium chloride is mixed with an aqueous solution of sodium nitrate. The complete ionic equation contains which of the following species (when balanced in standard form)?
A)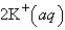
B)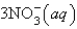
C)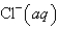
D)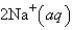
E)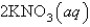
A)
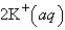
B)
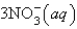
C)
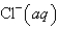
D)
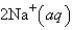
E)
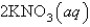

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 64 في هذه المجموعة.
فتح الحزمة
k this deck
25
The reaction HNO3(aq) + NaOH(aq)  H2O(l) + NaNO3(aq) is a(n) ________ reaction.
H2O(l) + NaNO3(aq) is a(n) ________ reaction.
A) acid-base
B) precipitation
C) oxidation-reduction
D) single replacement
E) none of these
 H2O(l) + NaNO3(aq) is a(n) ________ reaction.
H2O(l) + NaNO3(aq) is a(n) ________ reaction.A) acid-base
B) precipitation
C) oxidation-reduction
D) single replacement
E) none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 64 في هذه المجموعة.
فتح الحزمة
k this deck
26
An aqueous solution of potassium chloride is mixed with an aqueous solution of sodium nitrate. The net ionic equation contains which of the following species (when balanced in standard form)?
A)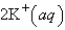
B)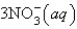
C)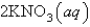
D)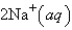
E) No net ionic equation exists for this reaction.
A)
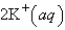
B)
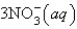
C)
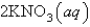
D)
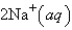
E) No net ionic equation exists for this reaction.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 64 في هذه المجموعة.
فتح الحزمة
k this deck
27
How many of the following are oxidation-reduction reactions?
(I) reaction of a metal with a nonmetal
(II) synthesis
(III) combustion
(IV) precipitation
(V) decomposition
A) 1
B) 2
C) 3
D) 4
E) 5
(I) reaction of a metal with a nonmetal
(II) synthesis
(III) combustion
(IV) precipitation
(V) decomposition
A) 1
B) 2
C) 3
D) 4
E) 5

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 64 في هذه المجموعة.
فتح الحزمة
k this deck
28
The reaction 2K(s) + Br2(l) 2KBr(s) is a(n) ______________ reaction.
A) precipitation
B) acid-base
C) oxidation-reduction
D) double-displacement
E) single-replacement
A) precipitation
B) acid-base
C) oxidation-reduction
D) double-displacement
E) single-replacement

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 64 في هذه المجموعة.
فتح الحزمة
k this deck
29
The equation 2C2H6 + 7O2 4CO2 + 6H2O is an oxidation-reduction reaction. and why?
A) True; the carbon is oxidized, and the oxygen is reduced.
B) True; the carbon is reduced, and the oxygen is oxidized.
C) True; the carbon is oxidized, and the hydrogen is reduced.
D) True; the oxygen is reduced, and the hydrogen is oxidized.
E) False
A) True; the carbon is oxidized, and the oxygen is reduced.
B) True; the carbon is reduced, and the oxygen is oxidized.
C) True; the carbon is oxidized, and the hydrogen is reduced.
D) True; the oxygen is reduced, and the hydrogen is oxidized.
E) False

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 64 في هذه المجموعة.
فتح الحزمة
k this deck
30
An aqueous solution of potassium chloride is mixed with an aqueous solution of sodium nitrate. The molecular equation contains which one of the following terms (when balanced in standard form)?
A) KCl(s)
B) KNO3(aq)
C) KNa(aq)
D) ClNO3(aq)
E) NaCl(s)
A) KCl(s)
B) KNO3(aq)
C) KNa(aq)
D) ClNO3(aq)
E) NaCl(s)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 64 في هذه المجموعة.
فتح الحزمة
k this deck
31
An aqueous solution of ammonium sulfide is allowed to react with an aqueous solution of barium chloride. What is the coefficient of the solid in the balanced equation (in standard form)?
A) 1
B) 2
C) 3
D) 4
E) 5
A) 1
B) 2
C) 3
D) 4
E) 5

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 64 في هذه المجموعة.
فتح الحزمة
k this deck
32
The reaction AgNO3(aq) + NaCl(aq) AgCl(s) + NaNO3(aq) is a(n) ______________ reaction.
A) precipitation
B) acid-base
C) oxidation-reduction
D) single-replacement
E) none of these
A) precipitation
B) acid-base
C) oxidation-reduction
D) single-replacement
E) none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 64 في هذه المجموعة.
فتح الحزمة
k this deck
33
Balance the complete ionic equation when aqueous barium hydroxide reacts with aqueous hydrochloric acid.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 64 في هذه المجموعة.
فتح الحزمة
k this deck
34
When the following equation is balanced, what is the coefficient for H2O? Ca(OH)2(aq) + H3PO4(aq) Ca3(PO4)2(s) + H2O(l)
A) 2
B) 3
C) 4
D) 6
E) 8
A) 2
B) 3
C) 4
D) 6
E) 8

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 64 في هذه المجموعة.
فتح الحزمة
k this deck
35
The scientist who discovered the essential nature of acids through solution conductivity studies is
A) Priestley
B) Boyle
C) Einstein
D) Mendeleev
E) Arrhenius
A) Priestley
B) Boyle
C) Einstein
D) Mendeleev
E) Arrhenius

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 64 في هذه المجموعة.
فتح الحزمة
k this deck
36
An aqueous solution of potassium chloride is mixed with an aqueous solution of sodium nitrate. Identify the solid in the balanced equation.
A) KCl
B) NaNO3
C) KNO3
D) NaCl
E) There is no solid formed when the two solutions are mixed.
A) KCl
B) NaNO3
C) KNO3
D) NaCl
E) There is no solid formed when the two solutions are mixed.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 64 في هذه المجموعة.
فتح الحزمة
k this deck
37
The complete ionic equation contains only those substances directly involved in reactions in aqueous solutions.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 64 في هذه المجموعة.
فتح الحزمة
k this deck
38
How many electrons are transferred in the following oxidation-reduction reaction? Zn(s) + 2AgNO3(aq) Zn(NO3)2(aq) + 2Ag(s)
A) 1
B) 2
C) 3
D) 4
E) 5
A) 1
B) 2
C) 3
D) 4
E) 5

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 64 في هذه المجموعة.
فتح الحزمة
k this deck
39
Write the molecular equation, the complete ionic equation, and the net ionic equation for the following reaction: Aqueous solutions of copper(II) nitrate and sodium hydroxide are mixed to form solid copper(II) hydroxide and aqueous sodium nitrate.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 64 في هذه المجموعة.
فتح الحزمة
k this deck
40
An aqueous solution of ammonium carbonate is allowed to react with an aqueous solution of nickel(II) chloride. Identify the solid in the balanced equation.
A) NiCO3
B) NH4Cl
C) NiCl2
D) (NH4)2CO3
E) There is no solid formed when the two solutions are mixed.
A) NiCO3
B) NH4Cl
C) NiCl2
D) (NH4)2CO3
E) There is no solid formed when the two solutions are mixed.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 64 في هذه المجموعة.
فتح الحزمة
k this deck
41
Classify the following reaction: 2Mg(s) + O2(g) 2MgO(s)
A) oxidation-reduction
B) combustion
C) synthesis
D) two of the above
E) a-c are all correct.
A) oxidation-reduction
B) combustion
C) synthesis
D) two of the above
E) a-c are all correct.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 64 في هذه المجموعة.
فتح الحزمة
k this deck
42
When a metal and a nonmetal react, the metal ______________ electrons and the nonmetal ______________ electrons.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 64 في هذه المجموعة.
فتح الحزمة
k this deck
43
Use the following choices to classify each reaction given below (more than one choice may apply).
-The equation 2Al(s) + 2Br2(l) 2AlBr3(s) is a(n) ______________ reaction.
A)oxidation-reduction and synthesis
B)oxidation-reduction only
C)synthesis only
D)decomposition
E)combustion
-The equation 2Al(s) + 2Br2(l) 2AlBr3(s) is a(n) ______________ reaction.
A)oxidation-reduction and synthesis
B)oxidation-reduction only
C)synthesis only
D)decomposition
E)combustion

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 64 في هذه المجموعة.
فتح الحزمة
k this deck
44
Use the following choices to classify each reaction given below (more than one choice may apply).
-H2SO4(aq) + Ba(OH)2(aq) 2H2O(l) + BaSO4(s)
A) oxidation-reduction
B) acid-base
C) precipitation
-H2SO4(aq) + Ba(OH)2(aq) 2H2O(l) + BaSO4(s)
A) oxidation-reduction
B) acid-base
C) precipitation

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 64 في هذه المجموعة.
فتح الحزمة
k this deck
45
Use the following choices to classify each reaction given below (more than one choice may apply).
-The equation 2Ag2O(s) 4Ag(s) + O2(g) is a(n) ______________ reaction.
A)oxidation-reduction
B)synthesis
C)decomposition
D)combustion
E)two of these
-The equation 2Ag2O(s) 4Ag(s) + O2(g) is a(n) ______________ reaction.
A)oxidation-reduction
B)synthesis
C)decomposition
D)combustion
E)two of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 64 في هذه المجموعة.
فتح الحزمة
k this deck
46
Use the following choices to classify each reaction given below (more than one choice may apply).
-Ca(s) + H2(g) CaH2(s)
A) oxidation-reduction
B) acid-base
C) precipitation
-Ca(s) + H2(g) CaH2(s)
A) oxidation-reduction
B) acid-base
C) precipitation

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 64 في هذه المجموعة.
فتح الحزمة
k this deck
47
Use the following choices to classify each reaction given below (more than one choice may apply).
-Na2SO4(aq) + Pb(NO3)2(aq) PbSO4(s) + 2NaNO3(aq)
A) oxidation-reduction
B) acid-base
C) precipitation
-Na2SO4(aq) + Pb(NO3)2(aq) PbSO4(s) + 2NaNO3(aq)
A) oxidation-reduction
B) acid-base
C) precipitation

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 64 في هذه المجموعة.
فتح الحزمة
k this deck
48
In what type of reaction is water always a product?
A) precipitation
B) acid-base
C) oxidation
D) decomposition
E) synthesis
A) precipitation
B) acid-base
C) oxidation
D) decomposition
E) synthesis

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 64 في هذه المجموعة.
فتح الحزمة
k this deck
49
A reaction that involves a transfer of electrons is called a(n) ______________ reaction.
A) precipitation
B) acid-base
C) oxidation-reduction
D) double-displacement
E) none of these
A) precipitation
B) acid-base
C) oxidation-reduction
D) double-displacement
E) none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 64 في هذه المجموعة.
فتح الحزمة
k this deck
50
Use the following choices to classify each reaction given below (more than one choice may apply).
-KBr(aq) + AgNO3(aq) AgBr(s) + KNO3(aq)
A) oxidation-reduction
B) acid-base
C) precipitation
-KBr(aq) + AgNO3(aq) AgBr(s) + KNO3(aq)
A) oxidation-reduction
B) acid-base
C) precipitation

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 64 في هذه المجموعة.
فتح الحزمة
k this deck
51
Classify the following reaction: HNO3(aq) + KOH(aq) KNO3(aq) +H2O(l)
A) oxidation-reduction
B) combustion
C) precipitation
D) acid-base
E) two of the above
A) oxidation-reduction
B) combustion
C) precipitation
D) acid-base
E) two of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 64 في هذه المجموعة.
فتح الحزمة
k this deck
52
Use the following choices to classify each reaction given below (more than one choice may apply).
-HNO3(aq) + NaOH(aq) H2O(l) + NaNO3(aq)
A) oxidation-reduction
B) acid-base
C) precipitation
-HNO3(aq) + NaOH(aq) H2O(l) + NaNO3(aq)
A) oxidation-reduction
B) acid-base
C) precipitation

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 64 في هذه المجموعة.
فتح الحزمة
k this deck
53
Use the following choices to classify each reaction given below (more than one choice may apply).
-ZnBr2(aq) + 2AgNO3(aq) Zn(NO3)2(aq) + 2AgBr(s)
A) oxidation-reduction
B) acid-base
C) precipitation
-ZnBr2(aq) + 2AgNO3(aq) Zn(NO3)2(aq) + 2AgBr(s)
A) oxidation-reduction
B) acid-base
C) precipitation

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 64 في هذه المجموعة.
فتح الحزمة
k this deck
54
Use the following choices to classify each reaction given below (more than one choice may apply).
-Zn(s) + 2HCl(aq) H2(g) + ZnCl2(aq)
A) oxidation-reduction
B) acid-base
C) precipitation
-Zn(s) + 2HCl(aq) H2(g) + ZnCl2(aq)
A) oxidation-reduction
B) acid-base
C) precipitation

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 64 في هذه المجموعة.
فتح الحزمة
k this deck
55
Use the following choices to classify each reaction given below (more than one choice may apply).
-CH4(g) + 2O2(g) CO2(g) + 2H2O(g)
A)oxidation-reduction
B)synthesis
C)decomposition
D)combustion
E)two of these
-CH4(g) + 2O2(g) CO2(g) + 2H2O(g)
A)oxidation-reduction
B)synthesis
C)decomposition
D)combustion
E)two of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 64 في هذه المجموعة.
فتح الحزمة
k this deck
56
Use the following choices to classify each reaction given below (more than one choice may apply).
-Zr(s) + O2(g) ZrO2(s)
A) oxidation-reduction
B) acid-base
C) precipitation
-Zr(s) + O2(g) ZrO2(s)
A) oxidation-reduction
B) acid-base
C) precipitation

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 64 في هذه المجموعة.
فتح الحزمة
k this deck
57
Use the following choices to classify each reaction given below (more than one choice may apply).
-HC2H3O2(aq) + CsOH(aq) H2O(l) + CsC2H3O2(aq)
A) oxidation-reduction
B) acid-base
C) precipitation
-HC2H3O2(aq) + CsOH(aq) H2O(l) + CsC2H3O2(aq)
A) oxidation-reduction
B) acid-base
C) precipitation

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 64 في هذه المجموعة.
فتح الحزمة
k this deck
58
Oxidation and reduction must occur simultaneously.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 64 في هذه المجموعة.
فتح الحزمة
k this deck
59
When the following equation is balanced in standard form, what is the coefficient in front of the H2O? C2H6(g) + O2(g)  CO2(g) + H2O(g)
CO2(g) + H2O(g)
A) 6
B) 2
C) 4
D) 1
E) 7
 CO2(g) + H2O(g)
CO2(g) + H2O(g)A) 6
B) 2
C) 4
D) 1
E) 7

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 64 في هذه المجموعة.
فتح الحزمة
k this deck
60
Which of the following statements is not true?
A) When a metal reacts with a nonmetal, an ionic compound is formed.
B) A metal-nonmetal reaction can always be assumed to be an oxidation-reduction reaction.
C) Two nonmetals can undergo an oxidation-reduction reaction.
D) When two nonmetals react, the compound formed is ionic.
E) A metal-nonmetal reaction involves electron transfer.
A) When a metal reacts with a nonmetal, an ionic compound is formed.
B) A metal-nonmetal reaction can always be assumed to be an oxidation-reduction reaction.
C) Two nonmetals can undergo an oxidation-reduction reaction.
D) When two nonmetals react, the compound formed is ionic.
E) A metal-nonmetal reaction involves electron transfer.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 64 في هذه المجموعة.
فتح الحزمة
k this deck
61
Use the following choices to classify each reaction given below (more than one choice may apply).
-P4(s) + 5O2(g) P4O10(s)
A) oxidation-reduction
B) acid-base
C) precipitation
-P4(s) + 5O2(g) P4O10(s)
A) oxidation-reduction
B) acid-base
C) precipitation

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 64 في هذه المجموعة.
فتح الحزمة
k this deck
62
Use the following choices to classify each reaction given below (more than one choice may apply).
-S8(s) + 12O2(g) 8SO3(g)
A) oxidation-reduction
B) acid-base
C) precipitation
-S8(s) + 12O2(g) 8SO3(g)
A) oxidation-reduction
B) acid-base
C) precipitation

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 64 في هذه المجموعة.
فتح الحزمة
k this deck
63
Use the following choices to classify each reaction given below (more than one choice may apply).
-C3H8(g) + 5O2(g) 3CO2(g) + 4H2O(g)
A) oxidation-reduction
B) acid-base
C) precipitation
-C3H8(g) + 5O2(g) 3CO2(g) + 4H2O(g)
A) oxidation-reduction
B) acid-base
C) precipitation

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 64 في هذه المجموعة.
فتح الحزمة
k this deck
64
Use the following choices to classify each reaction given below (more than one choice may apply).
-C(s) + O2(g) CO2(g)
A) oxidation-reduction
B) acid-base
C) precipitation
-C(s) + O2(g) CO2(g)
A) oxidation-reduction
B) acid-base
C) precipitation

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 64 في هذه المجموعة.
فتح الحزمة
k this deck








