Deck 23: Metallurgy and the Chemistry of Metals
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/115
العب
ملء الشاشة (f)
Deck 23: Metallurgy and the Chemistry of Metals
1
What elements are alloyed to make stainless steel?
A)Fe and C
B)Fe and Mn
C)Fe and Ni
D)Fe and Cr
E)Fe,Cr,and Ni
A)Fe and C
B)Fe and Mn
C)Fe and Ni
D)Fe and Cr
E)Fe,Cr,and Ni
Fe,Cr,and Ni
2
Electrometallurgy uses _________________ to separate a metal from its ore.
A)solid phase chemical properties
B)electrical processes
C)thermal processes
D)aqueous chemical processes
E)molten salt processes
A)solid phase chemical properties
B)electrical processes
C)thermal processes
D)aqueous chemical processes
E)molten salt processes
electrical processes
3
Which of these reactions represents the removal of silica from iron ore in a blast furnace?
A)SiO2(s)→ SiO2(g)
B)SiO2(s)+ CaO(s)→ CaSiO3(l)
C)SiO2(s)+ 4HF(g)→ SiF4(g)+ 2H2O(g)
D)SiO2(s)+ C(s)→ Si(l)+ CO2(g)
E)SiO2(s)+ CO(g)→ SiCO3(l)
A)SiO2(s)→ SiO2(g)
B)SiO2(s)+ CaO(s)→ CaSiO3(l)
C)SiO2(s)+ 4HF(g)→ SiF4(g)+ 2H2O(g)
D)SiO2(s)+ C(s)→ Si(l)+ CO2(g)
E)SiO2(s)+ CO(g)→ SiCO3(l)
SiO2(s)+ CaO(s)→ CaSiO3(l)
4
The process that selectively extracts a metal from its ore,by dissolving it,is called
A)roasting.
B)leaching.
C)smelting.
D)flotation.
E)hydration.
A)roasting.
B)leaching.
C)smelting.
D)flotation.
E)hydration.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
5
Alloying a metal is done to
A)make its extraction from its ore easier.
B)convert the metal to an oxide.
C)disguise the true identity of the metal.
D)prepare ultrapure metal samples.
E)enhance properties,like conductivity.
A)make its extraction from its ore easier.
B)convert the metal to an oxide.
C)disguise the true identity of the metal.
D)prepare ultrapure metal samples.
E)enhance properties,like conductivity.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
6
The debris accompanying a mineral is called
A)slag.
B)gangue.
C)ore.
D)halite.
E)ash.
A)slag.
B)gangue.
C)ore.
D)halite.
E)ash.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
7
The naturally occurring form of a metal that is concentrated enough to allow economical recovery of the metal is know as
A)an element.
B)a mineral.
C)gangue.
D)an ore.
E)an amalgam.
A)an element.
B)a mineral.
C)gangue.
D)an ore.
E)an amalgam.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
8
Pyrometallurgy uses __________________ to separate a metal from its ore.
A)solid phase chemical properties
B)electrical processes
C)thermal processes
D)aqueous chemical processes
E)explosives
A)solid phase chemical properties
B)electrical processes
C)thermal processes
D)aqueous chemical processes
E)explosives

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
9
Which one of these normally would not be obtained by chemical reduction?
A)Fe
B)Ti
C)Zn
D)Mg
E)Ag
A)Fe
B)Ti
C)Zn
D)Mg
E)Ag

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
10
In the Hall process,____________ is reduced ____________.
A)nickel; electrolytically
B)aluminum; electrolytically
C)nickel; by reaction with metallic sodium
D)aluminum; by reaction with metallic sodium
E)copper; electrolytically
A)nickel; electrolytically
B)aluminum; electrolytically
C)nickel; by reaction with metallic sodium
D)aluminum; by reaction with metallic sodium
E)copper; electrolytically

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
11
Nickel is often purified using
A)amalgamation.
B)the Hall process.
C)the Mond process.
D)pressurization.
E)a blast furnace.
A)amalgamation.
B)the Hall process.
C)the Mond process.
D)pressurization.
E)a blast furnace.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
12
Alloys of iron that contain 1.0-1.5% carbon and some manganese,phosphorus,silicon,and sulfur are called
A)steel.
B)cast iron.
C)coke.
D)pig iron.
E)hematite.
A)steel.
B)cast iron.
C)coke.
D)pig iron.
E)hematite.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
13
Hydrometallurgy uses __________________ to separate a metal from its ore.
A)solid phase chemical properties
B)electrical processes
C)thermal processes
D)aqueous chemical processes
E)molten salt processes
A)solid phase chemical properties
B)electrical processes
C)thermal processes
D)aqueous chemical processes
E)molten salt processes

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
14
Volatile impurities are removed from ores by means of
A)roasting.
B)amalgamation.
C)electrolysis.
D)flotation.
E)zone refining.
A)roasting.
B)amalgamation.
C)electrolysis.
D)flotation.
E)zone refining.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
15
Which is the principal reducing agent in a blast furnace?
A)CaO(s)
B)CaSiO3(l)
C)CO(g)
D)O2(g)
E)CO2(g)
A)CaO(s)
B)CaSiO3(l)
C)CO(g)
D)O2(g)
E)CO2(g)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
16
What is the source of most metals?
A)Industry
B)Synthesis
C)Saturated
D)Ocean floor
E)Minerals
A)Industry
B)Synthesis
C)Saturated
D)Ocean floor
E)Minerals

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
17
The most common source for the commercial production of sodium is called
A)sodalite.
B)limestone.
C)halite.
D)galena.
E)pyrite.
A)sodalite.
B)limestone.
C)halite.
D)galena.
E)pyrite.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
18
The most common source for the commercial production of aluminum is called
A)aluminite.
B)hematite.
C)galena.
D)cinnabar.
E)bauxite.
A)aluminite.
B)hematite.
C)galena.
D)cinnabar.
E)bauxite.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
19
The flotation process used in metallurgy involves
A)the roasting of sulfides.
B)separation of gangue from ore.
C)electrolytic reduction.
D)chemical reduction of a metal.
E)zone refining.
A)the roasting of sulfides.
B)separation of gangue from ore.
C)electrolytic reduction.
D)chemical reduction of a metal.
E)zone refining.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
20
The process of converting metal sulfides to metal oxides is called
A)roasting.
B)smelting.
C)flotation.
D)leaching.
E)sulfoxidation.
A)roasting.
B)smelting.
C)flotation.
D)leaching.
E)sulfoxidation.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
21
Cryolite,Na3AlF6,is used in the electrolysis of aluminum oxide because
A)it is a good source of fluoride ions.
B)it reduces the energy requirement of the process,due to its low melting point.
C)it provides a source of fluorine,an oxidizing agent.
D)it provides a source of sodium,a reducing agent.
E)it is very soluble in water.
A)it is a good source of fluoride ions.
B)it reduces the energy requirement of the process,due to its low melting point.
C)it provides a source of fluorine,an oxidizing agent.
D)it provides a source of sodium,a reducing agent.
E)it is very soluble in water.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
22
Which one of these elements would give a p-type semiconductor when added to a silicon crystal?
A)C
B)P
C)As
D)Ga
E)Sb
A)C
B)P
C)As
D)Ga
E)Sb

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
23
What is the major component in milk of magnesia?
A)Mg(OH)2
B)MgS
C)MgCl
D)MgSO4
E)MgCO3
A)Mg(OH)2
B)MgS
C)MgCl
D)MgSO4
E)MgCO3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
24
Which two compounds are produced by the Solvay process?
A)CaCO3 and CaO
B)CaCO3 and Na2CO3
C)NaHCO3 and NaCl
D)NH3 and NH4Cl
E)NaHCO3 and Na2CO3
A)CaCO3 and CaO
B)CaCO3 and Na2CO3
C)NaHCO3 and NaCl
D)NH3 and NH4Cl
E)NaHCO3 and Na2CO3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
25
What effect does increasing temperature have on the conductivities of semiconductors?
A)Increases
B)Decreases
C)No change
D)Cannot be predicted
A)Increases
B)Decreases
C)No change
D)Cannot be predicted

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
26
The alkali metals are isolated from nonaqueous systems.Why is this necessary?
A)The electrolysis of aqueous solutions of the alkali metals requires more energy than electrolysis of the molten salts.
B)The dissolved alkali earth halides are too reactive to be electrolyzed.
C)The aqueous metal ions are more difficult to reduce than water.
D)The reduction potentials of the alkali metals are more positive than the reduction potential of water.
E)The aqueous metal ions react violently with water.
A)The electrolysis of aqueous solutions of the alkali metals requires more energy than electrolysis of the molten salts.
B)The dissolved alkali earth halides are too reactive to be electrolyzed.
C)The aqueous metal ions are more difficult to reduce than water.
D)The reduction potentials of the alkali metals are more positive than the reduction potential of water.
E)The aqueous metal ions react violently with water.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
27
Calcium oxide is added to molten iron in the production of carbon steel in order to
A)convert silicon and phosphorus oxides to slag which can be decanted from the molten steel.
B)serve as a scrubber to remove sulfur dioxide from the gases leaving the furnace.
C)remove any traces of acid which could weaken the steel.
D)add a small amount of oxygen to the steel to prevent corrosion and increase its strength.
E)create gangue.
A)convert silicon and phosphorus oxides to slag which can be decanted from the molten steel.
B)serve as a scrubber to remove sulfur dioxide from the gases leaving the furnace.
C)remove any traces of acid which could weaken the steel.
D)add a small amount of oxygen to the steel to prevent corrosion and increase its strength.
E)create gangue.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
28
Seawater is a good source of which of the following metals?
A)Cu
B)Au
C)Mg
D)Cl
E)Ca
A)Cu
B)Au
C)Mg
D)Cl
E)Ca

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
29
According to the band theory,which of these statements provide(s)an explanation for the high electrical conductivity of metals? I.a completely filled conduction band
II)a valence band overlapping an empty conduction band
III)a filled valence band
IV)a large gap between the valence band and the conduction band
A)II
B)I and III
C)III
D)III and IV
E)IV
II)a valence band overlapping an empty conduction band
III)a filled valence band
IV)a large gap between the valence band and the conduction band
A)II
B)I and III
C)III
D)III and IV
E)IV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
30
What are two raw materials used in the Solvay process?
A)NaHCO3 and NaCl
B)CaCO3 and Na2CO3
C)NaCl and NaCO3
D)NH3 and NaCl
E)Na2CO3 and NH4Cl
A)NaHCO3 and NaCl
B)CaCO3 and Na2CO3
C)NaCl and NaCO3
D)NH3 and NaCl
E)Na2CO3 and NH4Cl

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
31
According to the band theory,a band is
A)an elastic force that holds electrons close to atoms in an insulator.
B)the energy gap associated with semiconductors.
C)a large number of molecular orbitals that are close together in energy.
D)a large number of atoms in a crystal.
A)an elastic force that holds electrons close to atoms in an insulator.
B)the energy gap associated with semiconductors.
C)a large number of molecular orbitals that are close together in energy.
D)a large number of atoms in a crystal.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
32
Basic properties are characteristic of all alkaline earth metal oxides except one.Which is the exception?
A)BeO
B)MgO
C)SO2
D)B2O3
E)CaO
A)BeO
B)MgO
C)SO2
D)B2O3
E)CaO

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
33
Where on the periodic table are the most reactive metals located?
A)Group IA (alkali metals)
B)Group IA (alkaline earth metals)
C)Group IIA (alkali metals)
D)Group IIA (alkaline earth metals)
E)Group VIIA (halogens)
A)Group IA (alkali metals)
B)Group IA (alkaline earth metals)
C)Group IIA (alkali metals)
D)Group IIA (alkaline earth metals)
E)Group VIIA (halogens)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
34
What is the major component in limestone?
A)NaNO3
B)KCl
C)CaCl
D)BaSO4
E)CaCO3
A)NaNO3
B)KCl
C)CaCl
D)BaSO4
E)CaCO3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
35
Which of these elements when doped into silicon would give an n-type semiconductor?
A)C
B)Ga
C)P
D)Ge
E)B
A)C
B)Ga
C)P
D)Ge
E)B

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
36
What is the chemical formula for gypsum?
A)CaSO4• 2H2O
B)CaF2
C)MgSO4• 5H2O
D)MgSO4• 7H2O
E)CaCl2
A)CaSO4• 2H2O
B)CaF2
C)MgSO4• 5H2O
D)MgSO4• 7H2O
E)CaCl2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
37
In the production of potassium metal,the source of electrons in the reduction of K+ ions is
A)H2(g).
B)Na(g).
C)CO(g).
D)CaO(s).
E)electrolysis.
A)H2(g).
B)Na(g).
C)CO(g).
D)CaO(s).
E)electrolysis.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
38
What is the name of the relationship that exists because of the similar properties of carbon to phosphorus and nitrogen to sulfur?
A)A diagonal relationship
B)The periodic law
C)Amphoterism
D)An isoelectronic relationship
E)An allotropic relationship
A)A diagonal relationship
B)The periodic law
C)Amphoterism
D)An isoelectronic relationship
E)An allotropic relationship

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
39
Electrolysis is used as the last step in isolating pure __________________.
A)iron
B)boron
C)aluminum
D)selenium
E)carbon
A)iron
B)boron
C)aluminum
D)selenium
E)carbon

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
40
In n-type semiconductors
A)the energy gap between the valence band and the conduction band is very large.
B)impurities that donate electrons are added to provide conduction electrons.
C)a valence band overlaps the empty conduction band.
D)impurities that provide "positive holes" are added to a pure semiconductor.
A)the energy gap between the valence band and the conduction band is very large.
B)impurities that donate electrons are added to provide conduction electrons.
C)a valence band overlaps the empty conduction band.
D)impurities that provide "positive holes" are added to a pure semiconductor.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
41
Which of these ions is most likely to substitute for Ca2+ in the human body?
A)Cl-
B)Sr2+
C)K+
D)S2-
E)Pb2+
A)Cl-
B)Sr2+
C)K+
D)S2-
E)Pb2+

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
42
The mineral cryolite,Na3AlF6,is used in the Hall process for aluminum production as
A)the source of aluminum (the ore).
B)a chemical reducing agent.
C)a material that forms a slag and thus removes impurities.
D)a solvent for alumina,Al2O3.
A)the source of aluminum (the ore).
B)a chemical reducing agent.
C)a material that forms a slag and thus removes impurities.
D)a solvent for alumina,Al2O3.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
43
In the production of aluminum,bauxite is heated with sodium hydroxide solution to _____.
A)remove titanium(IV)oxide
B)remove silica
C)remove iron oxides
D)remove potassium
E)remove fluorides
A)remove titanium(IV)oxide
B)remove silica
C)remove iron oxides
D)remove potassium
E)remove fluorides

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
44
Which mineral is not a carbonate?
A)Witherite
B)Sylvite
C)Smithsonite
D)Cerussite
E)Calcite
A)Witherite
B)Sylvite
C)Smithsonite
D)Cerussite
E)Calcite

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
45
In gunpowder,which component is present in the highest percent by mass?
A)Wood charcoal
B)Chile salt peter
C)Potassium nitrate
D)Sulfur
E)Ammonia
A)Wood charcoal
B)Chile salt peter
C)Potassium nitrate
D)Sulfur
E)Ammonia

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
46
Which reaction is a good small-scale laboratory method for the preparation of hydrogen?
A)CH4(g)+ H2O(g)→ CO(g)+ 3H2(g)
B)3FeCl2 (s)+ 4H2O(g)→ Fe3O4(s)+ 6HCl(g)+ H2(g)
C)NaH(s)+ H2O(l)→ NaOH(aq)+ H2(g)
D)CO(g)+ H2O(g)→ CO2(g)+ H2(g)
E)None of these methods is useful.
A)CH4(g)+ H2O(g)→ CO(g)+ 3H2(g)
B)3FeCl2 (s)+ 4H2O(g)→ Fe3O4(s)+ 6HCl(g)+ H2(g)
C)NaH(s)+ H2O(l)→ NaOH(aq)+ H2(g)
D)CO(g)+ H2O(g)→ CO2(g)+ H2(g)
E)None of these methods is useful.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
47
The Downs cell is used in the production of
A)copper.
B)hydrogen.
C)iron.
D)magnesium.
E)sodium.
A)copper.
B)hydrogen.
C)iron.
D)magnesium.
E)sodium.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
48
What is the molecular shape of AlF3?
A)Tetrahedral
B)Linear
C)Trigonal pyramidal
D)Square planar
E)Trigonal planar
A)Tetrahedral
B)Linear
C)Trigonal pyramidal
D)Square planar
E)Trigonal planar

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
49
The Hall process involves the reduction of Al2O3 to aluminum by
A)carbon (coke).
B)carbon monoxide.
C)molecular hydrogen.
D)sodium.
E)electrolysis.
A)carbon (coke).
B)carbon monoxide.
C)molecular hydrogen.
D)sodium.
E)electrolysis.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
50
Which is an example of a basic oxide?
A)SO3(g)
B)BaO(s)
C)Al2O3(s)
D)Fe2O3(s)
E)SiO2(s)
A)SO3(g)
B)BaO(s)
C)Al2O3(s)
D)Fe2O3(s)
E)SiO2(s)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
51
Magnesium reacts with dilute hydrochloric acid according to which of these equations?
A)Mg + 2HCl → 2H+ + MgCl
B)Mg + 2HCl → H2 + MgCl2
C)Mg + 2HCl → Cl2 + MgH2
D)Mg + 2HCl → Cl2 + Mg2+ + 2H+
A)Mg + 2HCl → 2H+ + MgCl
B)Mg + 2HCl → H2 + MgCl2
C)Mg + 2HCl → Cl2 + MgH2
D)Mg + 2HCl → Cl2 + Mg2+ + 2H+

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
52
The final step of the purification of copper involves electrorefining in which copper is separated from nickel and iron by being reduced at the cathode of a cell.Why are nickel and iron not reduced?
A)Their reduction potentials are more positive than copper's.
B)Their reduction potentials are more negative than copper's.
C)They cannot be deposited on a copper electrode.
D)Their reduction potentials are more negative than water's.
E)Their reduction requires large overvoltages.
A)Their reduction potentials are more positive than copper's.
B)Their reduction potentials are more negative than copper's.
C)They cannot be deposited on a copper electrode.
D)Their reduction potentials are more negative than water's.
E)Their reduction requires large overvoltages.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
53
The process used to produce metals with a purity of more than 99.99% is called
A)zone refining.
B)electrorefining.
C)distillation.
D)sublimation.
E)alloying.
A)zone refining.
B)electrorefining.
C)distillation.
D)sublimation.
E)alloying.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
54
Aluminum is an active metal,but does not corrode as iron does because
A)Al does not react with O2 .
B)a protective layer of Al2O3 forms on the metal surface.
C)Al is harder to oxidize than is Fe.
D)the enthalpy of formation of aluminum oxide is negative.
E)aluminum has a high tensile strength.
A)Al does not react with O2 .
B)a protective layer of Al2O3 forms on the metal surface.
C)Al is harder to oxidize than is Fe.
D)the enthalpy of formation of aluminum oxide is negative.
E)aluminum has a high tensile strength.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
55
_____ consists of potassium nitrate,wood charcoal,and sulfur.
A)Salt peter
B)Chile salt peter
C)Carborundum
D)Gunpowder
E)Anglesite
A)Salt peter
B)Chile salt peter
C)Carborundum
D)Gunpowder
E)Anglesite

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
56
Which metal's commercial source is seawater?
A)Cs
B)K
C)Mg
D)Ba
E)Li
A)Cs
B)K
C)Mg
D)Ba
E)Li

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
57
Which is a covalent oxide?
A)CaO
B)Na2O
C)Li2O
D)Cs2O
E)BeO
A)CaO
B)Na2O
C)Li2O
D)Cs2O
E)BeO

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
58
The Hall process refers to
A)the production of aluminum by electrolysis.
B)the recovery of sulfur from underground deposits.
C)the manufacture of sulfuric acid.
D)the production of ammonia from nitrogen and hydrogen gases.
E)the isolation of Al2O3 from bauxite.
A)the production of aluminum by electrolysis.
B)the recovery of sulfur from underground deposits.
C)the manufacture of sulfuric acid.
D)the production of ammonia from nitrogen and hydrogen gases.
E)the isolation of Al2O3 from bauxite.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
59
Which undergoes electrolysis to form calcium metal?
A)Ca(OH)2
B)Ca(NO3)2
C)CaI2
D)CaF2
E)CaCl2
A)Ca(OH)2
B)Ca(NO3)2
C)CaI2
D)CaF2
E)CaCl2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
60
Which statement is not correct?
A)Aluminum has a low density versus other metals.
B)Al(OH)3 is an amphoteric compound.
C)Metallic aluminum is toxic to humans.
D)Aluminum is very reactive with oxygen.
E)Metallic aluminum conducts electricity.
A)Aluminum has a low density versus other metals.
B)Al(OH)3 is an amphoteric compound.
C)Metallic aluminum is toxic to humans.
D)Aluminum is very reactive with oxygen.
E)Metallic aluminum conducts electricity.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
61
Which mineral is not a silicate?
A)Zircon
B)Beryl
C)Smithsonite
D)Albite
E)Talc
A)Zircon
B)Beryl
C)Smithsonite
D)Albite
E)Talc

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
62
Group 1A elements are called alkaline earth metals.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
63
Which mineral contains calcium as a major element?
A)Cinnabar
B)Corundum
C)Hematite
D)Hydroxyapatite
E)Talc
A)Cinnabar
B)Corundum
C)Hematite
D)Hydroxyapatite
E)Talc

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
64
What is the chemical formula for phosphate rock?
A)Na3PO4
B)Na3P
C)Mg3(PO4)2
D)AlPO4
E)Ca3(PO4)2
A)Na3PO4
B)Na3P
C)Mg3(PO4)2
D)AlPO4
E)Ca3(PO4)2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
65
What is the name of the process used to separate the minerals from the waste in ore?
A)Gangue
B)Mining
C)Fetching
D)Flotation
E)Mineral drawing
A)Gangue
B)Mining
C)Fetching
D)Flotation
E)Mineral drawing

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
66
Which diagram best corresponds to the energy level diagram of a semiconductor?
A)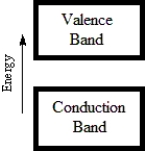
B)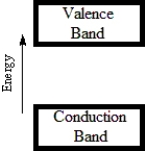
C)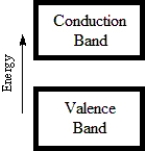
D)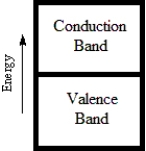
E)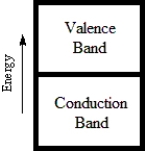
A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
67
Which ion is not found in sea water in a concentration high enough to be an industrial source of the metal?
A)Ca2+
B)Mg2+
C)Na+
D)K+
E)Ag+
A)Ca2+
B)Mg2+
C)Na+
D)K+
E)Ag+

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
68
What is the chemical formula for cinnabar?
A)HgS
B)SnBr
C)SnO2
D)TiO2
E)SnBr4
A)HgS
B)SnBr
C)SnO2
D)TiO2
E)SnBr4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
69
Which mineral is a silicate?
A)Cinnabar
B)Corundum
C)Smithsonite
D)Hydroxyapatite
E)Talc
A)Cinnabar
B)Corundum
C)Smithsonite
D)Hydroxyapatite
E)Talc

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
70
The following electrolytic cell is a representation of the process used to purify copper metal.How does it work? 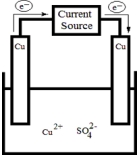 .
.
A)Impure copper is oxidized at the anode,and pure copper is reduced at the cathode.Impurities dissolve into solution.
B)Impure copper is oxidized at the cathode,and pure copper is reduced at the anode.Impurities dissolve into solution.
C)Impure copper is oxidized at the anode,and pure copper dissolves into solution.Impurities remain on the cathode.
D)Impure copper is oxidized at the cathode,and pure copper dissolves into solution.Impurities remain on the anode.
E)Impure copper precipitates from solution onto the cathode,and pure copper remains on the anode.
 .
.A)Impure copper is oxidized at the anode,and pure copper is reduced at the cathode.Impurities dissolve into solution.
B)Impure copper is oxidized at the cathode,and pure copper is reduced at the anode.Impurities dissolve into solution.
C)Impure copper is oxidized at the anode,and pure copper dissolves into solution.Impurities remain on the cathode.
D)Impure copper is oxidized at the cathode,and pure copper dissolves into solution.Impurities remain on the anode.
E)Impure copper precipitates from solution onto the cathode,and pure copper remains on the anode.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
71
What is the chemical formula for talc?
A)NaNO3
B)Mg3(PO4)2
C)CaCO3
D)Mg3(Si4O10)(OH)2
E)Ca3(PO4)2
A)NaNO3
B)Mg3(PO4)2
C)CaCO3
D)Mg3(Si4O10)(OH)2
E)Ca3(PO4)2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
72
What type of metal is generally separated using a strong electromagnet?
A)ferromagnetic
B)oxide
C)alkaline
D)uncombined
A)ferromagnetic
B)oxide
C)alkaline
D)uncombined

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
73
What is the chemical formula for limestone?
A)LiOH
B)Li2CO3
C)CaCO3
D)BaCO3
E)Ca3(PO4)2
A)LiOH
B)Li2CO3
C)CaCO3
D)BaCO3
E)Ca3(PO4)2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
74
Which diagram best corresponds to the energy level diagram of a metal?
A)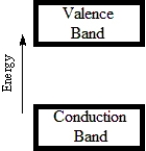
B)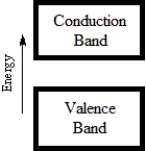
C)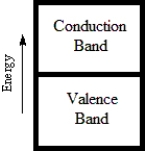
D)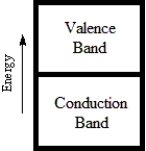
A)

B)

C)

D)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
75
A fractional distillation method called the Mond process is used to purify nickel.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
76
The first step in the purification of a metal M is represented by the following figure.To which process does the figure correspond,and which metal is usually purified by this process? 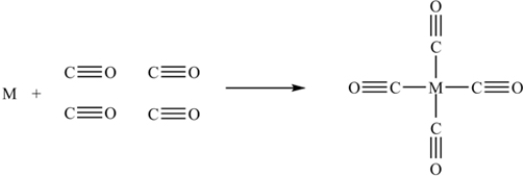
A)The Mond process,for the purification of Zn
B)The Mond process,for the purification of Ni
C)The Hall process,for the purification of Al
D)The Hall process,for the purification of Cr
E)The zone refining process,for the purification of Fe

A)The Mond process,for the purification of Zn
B)The Mond process,for the purification of Ni
C)The Hall process,for the purification of Al
D)The Hall process,for the purification of Cr
E)The zone refining process,for the purification of Fe

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
77
Which is NOT a chemical reaction that occurs in a blast furnace in the production of iron?
A)CaCO3(s)→ CaO(s)+ CO2(g)
B)3Fe2O3(s)+ CO(g)→ 2Fe3O4(s)+ CO2(g)
C)2FeO(s)+ CO(g)→ Fe2O3(s)+ C(s)
D)Fe3O4(s)+ CO(g)→ 3FeO(s)+ CO2(g)
E)FeO(s)+ CO(g)→ Fe(l)+ CO2(g)
A)CaCO3(s)→ CaO(s)+ CO2(g)
B)3Fe2O3(s)+ CO(g)→ 2Fe3O4(s)+ CO2(g)
C)2FeO(s)+ CO(g)→ Fe2O3(s)+ C(s)
D)Fe3O4(s)+ CO(g)→ 3FeO(s)+ CO2(g)
E)FeO(s)+ CO(g)→ Fe(l)+ CO2(g)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
78
Which is the best representation for a solution composed of sodium dissolved in liquid ammonia? (Ammonia molecules have been omitted for clarity.)
A)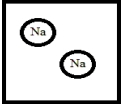
B)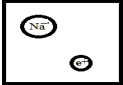
C)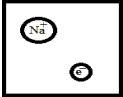
D)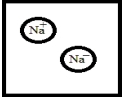
E)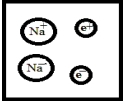
A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
79
For the production of metallic chromium at high temperatures,which is NOT an appropriate choice for the metal M? Cr2O3(s)+ 3M(l)→ 2Cr(l)+ 3MO(s)
A)Mg
B)Sn
C)Ca
D)Cu
E)Sr
A)Mg
B)Sn
C)Ca
D)Cu
E)Sr

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck
80
When mercury combines with other metals,what is the name given to the species formed?
A)Mercury complex
B)Mercury poison
C)Amalgam
D)Mineralization
E)Mercuration
A)Mercury complex
B)Mercury poison
C)Amalgam
D)Mineralization
E)Mercuration

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 115 في هذه المجموعة.
فتح الحزمة
k this deck








