Deck 8: Chemical Bonding I Basic Concepts
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/95
العب
ملء الشاشة (f)
Deck 8: Chemical Bonding I Basic Concepts
1
How many dots does the Lewis dot symbol for magnesium have around it?
A)1
B)2
C)0
D)3
E)7
A)1
B)2
C)0
D)3
E)7
2
2
The Lewis dot symbol for the chloride ion is
A)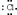
B) -
-
C) -
-
D)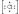
E)Cl-
A)
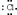
B)
 -
-C)
 -
-D)
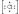
E)Cl-
 -
- 3
How many dots does the Lewis dot symbol for carbon have around it?
A)4
B)2
C)6
D)3
E)7
A)4
B)2
C)6
D)3
E)7
4
4
The Lewis dot symbol for the calcium ion is
A) 2+
2+
B)
C) 2+
2+
D)Ca2+
E)Ca
A)
 2+
2+B)

C)
 2+
2+D)Ca2+
E)Ca

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 95 في هذه المجموعة.
فتح الحزمة
k this deck
5
Which of these compounds is most likely to be ionic?
A)KF
B)CCl4
C)CS2
D)CO2
E)ICl
A)KF
B)CCl4
C)CS2
D)CO2
E)ICl

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 95 في هذه المجموعة.
فتح الحزمة
k this deck
6
The Lewis dot symbol consists of the symbol for the element surrounded by dot(s).What does the symbol represent?
A)Electron configuration
B)Valence electrons
C)Atomic number
D)Atomic mass
E)Nucleus and core electrons
A)Electron configuration
B)Valence electrons
C)Atomic number
D)Atomic mass
E)Nucleus and core electrons

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 95 في هذه المجموعة.
فتح الحزمة
k this deck
7
Which of the following contains ionic bonding?
A)CO
B)SrF2
C)Al
D)OCl2
E)HCl
A)CO
B)SrF2
C)Al
D)OCl2
E)HCl

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 95 في هذه المجموعة.
فتح الحزمة
k this deck
8
Which of these compounds is most likely to be ionic?
A)NCl3
B)BaCl2
C)CO
D)SO2
E)SF4
A)NCl3
B)BaCl2
C)CO
D)SO2
E)SF4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 95 في هذه المجموعة.
فتح الحزمة
k this deck
9
How many dots does the Lewis dot symbol for oxygen have around it?
A)4
B)2
C)6
D)3
E)7
A)4
B)2
C)6
D)3
E)7

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 95 في هذه المجموعة.
فتح الحزمة
k this deck
10
The Lewis dot symbol for the S2- ion is
A)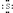
B) 2-
2-
C)S2-
D)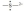
E)
A)
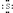
B)
 2-
2-C)S2-
D)
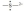
E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 95 في هذه المجموعة.
فتح الحزمة
k this deck
11
Select the element whose Lewis symbol is correct.
A)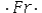
B)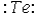
C)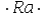
D)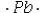
E)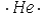
A)
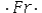
B)
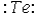
C)
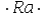
D)
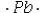
E)
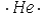

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 95 في هذه المجموعة.
فتح الحزمة
k this deck
12
How many dots does the Lewis dot symbol for chlorine have around it?
A)1
B)2
C)5
D)7
E)17
A)1
B)2
C)5
D)7
E)17

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 95 في هذه المجموعة.
فتح الحزمة
k this deck
13
The Lewis dot symbol for the a lead atom is
A)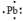
B)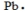
C)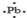
D)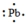
E)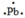
A)
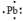
B)
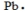
C)
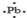
D)
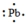
E)
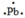

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 95 في هذه المجموعة.
فتح الحزمة
k this deck
14
Select the element whose Lewis symbol is correct.
A)•Ga•
B)•Al•
C)Al•
D)
•
•
Tl
•
•
A)•Ga•
B)•Al•
C)Al•
D)
•
•
Tl
•
•

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 95 في هذه المجموعة.
فتح الحزمة
k this deck
15
Which of these compounds is most likely to be ionic?
A)GaAs
B)SrBr2
C)NO2
D)CBr4
E)H2O
A)GaAs
B)SrBr2
C)NO2
D)CBr4
E)H2O

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 95 في هذه المجموعة.
فتح الحزمة
k this deck
16
Which of the following is an ionic compound?
A)H2S
B)NH3
C)I2
D)KI
E)CCl4
A)H2S
B)NH3
C)I2
D)KI
E)CCl4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 95 في هذه المجموعة.
فتح الحزمة
k this deck
17
Which of these pairs of elements would be most likely to form an ionic compound?
A)Cl and I
B)Al and K
C)Cl and Mg
D)C and S
E)Al and Mg
A)Cl and I
B)Al and K
C)Cl and Mg
D)C and S
E)Al and Mg

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 95 في هذه المجموعة.
فتح الحزمة
k this deck
18
The Lewis dot symbol consists of the symbol for the element surrounded by dot(s).What does the dot or dots represent?
A)Electron configuration
B)Valence electrons
C)Atomic number
D)Atomic mass
E)Core electrons
A)Electron configuration
B)Valence electrons
C)Atomic number
D)Atomic mass
E)Core electrons

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 95 في هذه المجموعة.
فتح الحزمة
k this deck
19
How many dots does the Lewis dot symbol for sodium have around it?
A)1
B)2
C)0
D)3
E)7
A)1
B)2
C)0
D)3
E)7

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 95 في هذه المجموعة.
فتح الحزمة
k this deck
20
How many dots does the Lewis dot symbol for argon have around it?
A)1
B)2
C)4
D)6
E)8
A)1
B)2
C)4
D)6
E)8

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 95 في هذه المجموعة.
فتح الحزمة
k this deck
21
Which molecule has the largest dipole moment?
A)HF
B)HI
C)HBr
D)HCl
E)All of the molecules have the same dipole moment.
A)HF
B)HI
C)HBr
D)HCl
E)All of the molecules have the same dipole moment.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 95 في هذه المجموعة.
فتح الحزمة
k this deck
22
Select the compound with the highest (i.e.,most negative)lattice energy.
A)CaS(s)
B)BaO(s)
C)NaI(s)
D)LiBr(s)
E)MgO(s)
A)CaS(s)
B)BaO(s)
C)NaI(s)
D)LiBr(s)
E)MgO(s)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 95 في هذه المجموعة.
فتح الحزمة
k this deck
23
Which of the following is a covalent compound?
A)Na2O
B)CaCl2
C)Cl2O
D)CsCl
E)Al2O3
A)Na2O
B)CaCl2
C)Cl2O
D)CsCl
E)Al2O3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 95 في هذه المجموعة.
فتح الحزمة
k this deck
24
What types of elements undergo ionic bonding?
A)two metals
B)a nonmetal and a metal
C)two nonmetals
D)two Group 1A elements
E)two noble gases
A)two metals
B)a nonmetal and a metal
C)two nonmetals
D)two Group 1A elements
E)two noble gases

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 95 في هذه المجموعة.
فتح الحزمة
k this deck
25
Which of these compounds is most likely to be covalent?
A)Rb2S
B)SrCl2
C)CS2
D)CaO
E)MgI2
A)Rb2S
B)SrCl2
C)CS2
D)CaO
E)MgI2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 95 في هذه المجموعة.
فتح الحزمة
k this deck
26
The Lewis structure for a chlorate ion,ClO3-,should show ____ single bond(s),____ double bond(s),and ____ lone pair(s).
A)2,1,10
B)3,0,9
C)2,1,8
D)3,0,10
E)2,1,9
A)2,1,10
B)3,0,9
C)2,1,8
D)3,0,10
E)2,1,9

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 95 في هذه المجموعة.
فتح الحزمة
k this deck
27
The Lewis dot symbol consists of the symbol for the element surrounded by dot(s).What does the symbol represent?
A)Electron configuration
B)Valence electrons
C)Atomic number
D)Atomic mass
E)Nucleus and core electrons
A)Electron configuration
B)Valence electrons
C)Atomic number
D)Atomic mass
E)Nucleus and core electrons

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 95 في هذه المجموعة.
فتح الحزمة
k this deck
28
The lattice energy for ionic crystals increases as the charge on the ions _____________ and the size of the ions __________________ .
A)increases,increases
B)increases,decreases
C)decreases,increases
D)decreases,decreases
E)None of these is generally correct.
A)increases,increases
B)increases,decreases
C)decreases,increases
D)decreases,decreases
E)None of these is generally correct.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 95 في هذه المجموعة.
فتح الحزمة
k this deck
29
Which of these compounds is most likely to be covalent?
A)KF
B)CaCl2
C)SF4
D)Al2O3
E)CaSO4
A)KF
B)CaCl2
C)SF4
D)Al2O3
E)CaSO4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 95 في هذه المجموعة.
فتح الحزمة
k this deck
30
Which of these Lewis structures is incorrect?
A)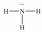
B)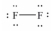
C)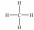
D)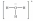
E)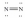
A)

B)
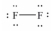
C)

D)

E)
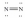

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 95 في هذه المجموعة.
فتح الحزمة
k this deck
31
Select the compound with the lowest (i.e.,least negative)lattice energy.
A)CsBr(s)
B)NaCl(s)
C)SrO(s)
D)CaO(s)
E)KBr(s)
A)CsBr(s)
B)NaCl(s)
C)SrO(s)
D)CaO(s)
E)KBr(s)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 95 في هذه المجموعة.
فتح الحزمة
k this deck
32
Which of the following contains ionic bonding?
A)CO
B)SrF2
C)Al
D)OCl2
E)HCl
A)CO
B)SrF2
C)Al
D)OCl2
E)HCl

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 95 في هذه المجموعة.
فتح الحزمة
k this deck
33
Which of these ionic solids would have the largest lattice energy?
A)NaCl
B)NaF
C)CaBr2
D)CsI
E)CaCl2
A)NaCl
B)NaF
C)CaBr2
D)CsI
E)CaCl2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 95 في هذه المجموعة.
فتح الحزمة
k this deck
34
Which of these ionic solids would have the largest lattice energy?
A)SrO
B)NaF
C)CaBr2
D)CsI
E)BaSO4
A)SrO
B)NaF
C)CaBr2
D)CsI
E)BaSO4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 95 في هذه المجموعة.
فتح الحزمة
k this deck
35
What types of elements undergo covalent bonding?
A)a metal and a noble gas
B)a nonmetal and a metal
C)two nonmetals
D)two Group 1A elements
E)an actinide
A)a metal and a noble gas
B)a nonmetal and a metal
C)two nonmetals
D)two Group 1A elements
E)an actinide

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 95 في هذه المجموعة.
فتح الحزمة
k this deck
36
How many lone pairs of electrons need to be added to complete this Lewis structure? 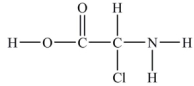
A)5
B)8
C)6
D)1
E)16

A)5
B)8
C)6
D)1
E)16

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 95 في هذه المجموعة.
فتح الحزمة
k this deck
37
Which of the following contains covalent bonds?
A)BaO
B)IBr
C)Mg
D)LiBr
E)Cu
A)BaO
B)IBr
C)Mg
D)LiBr
E)Cu

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 95 في هذه المجموعة.
فتح الحزمة
k this deck
38
The total number of bonding electrons in a molecule of formaldehyde (H2CO)is
A)3.
B)4.
C)6.
D)8.
E)18.
A)3.
B)4.
C)6.
D)8.
E)18.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 95 في هذه المجموعة.
فتح الحزمة
k this deck
39
The Lewis dot symbol consists of the symbol for the element surrounded by dot(s).What does the dot or dots represent?
A)Electron configuration
B)Valence electrons
C)Atomic number
D)Atomic mass
E)Core electrons
A)Electron configuration
B)Valence electrons
C)Atomic number
D)Atomic mass
E)Core electrons

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 95 في هذه المجموعة.
فتح الحزمة
k this deck
40
Which of these compounds is most likely to be covalent?
A)CsOH
B)NF3
C)Sr(NO3)2
D)CaO
E)LiF
A)CsOH
B)NF3
C)Sr(NO3)2
D)CaO
E)LiF

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 95 في هذه المجموعة.
فتح الحزمة
k this deck
41
Select the correct Lewis structure for nitrogen trifluoride,NF3.
A)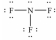
B)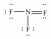
C)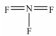
D)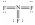
E)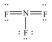
A)

B)
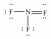
C)
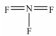
D)

E)
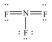

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 95 في هذه المجموعة.
فتح الحزمة
k this deck
42
Select the correct Lewis structure for NOCl,a reactive material used as an ionizing solvent.
A)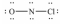
B)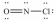
C)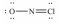
D)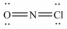
E)None of these choices is correct.
A)
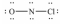
B)
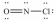
C)
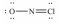
D)
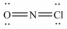
E)None of these choices is correct.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 95 في هذه المجموعة.
فتح الحزمة
k this deck
43
The formal charge on Cl in the structure shown for the perchlorate ion is _____. 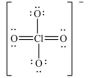
A)-2
B)-1
C)0
D)+1
E)+2

A)-2
B)-1
C)0
D)+1
E)+2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 95 في هذه المجموعة.
فتح الحزمة
k this deck
44
The number of resonance structures for the nitrate ion that satisfy the octet rule is
A)1.
B)2.
C)3.
D)4.
E)none of these
A)1.
B)2.
C)3.
D)4.
E)none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 95 في هذه المجموعة.
فتح الحزمة
k this deck
45
In the following Lewis structure for ClO3F,chlorine has a formal charge of ____ and an oxidation number of ____. 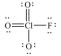
A)7,7
B)7,-1
C)1,1
D)1,-1
E)1,7

A)7,7
B)7,-1
C)1,1
D)1,-1
E)1,7

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 95 في هذه المجموعة.
فتح الحزمة
k this deck
46
In the Lewis structure of the iodate ion,IO3-,that satisfies the octet rule,the formal charge on the central iodine atom is
A)2.
B)1.
C)0.
D)-1.
E)-2.
A)2.
B)1.
C)0.
D)-1.
E)-2.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 95 في هذه المجموعة.
فتح الحزمة
k this deck
47
Which pair of Lewis structures is not a pair of resonance structures?
A)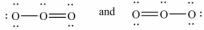
B)
C)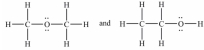
D)All of these pairs are resonance structures of the same species.
E)None of these pairs is resonance structures of the same species.
A)
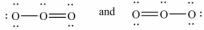
B)

C)
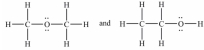
D)All of these pairs are resonance structures of the same species.
E)None of these pairs is resonance structures of the same species.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 95 في هذه المجموعة.
فتح الحزمة
k this deck
48
In the resonance structure of sulfur dioxide,the sulfur atom has one single bond and one double bond.What is the formal charge of this sulfur atom?
A)0
B)+1
C)-1
D)+2
E)-2
A)0
B)+1
C)-1
D)+2
E)-2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 95 في هذه المجموعة.
فتح الحزمة
k this deck
49
Which one of the following Lewis structures is definitely incorrect?
A)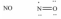
B)
C)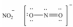
D)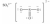
E)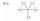
A)
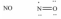
B)

C)
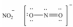
D)

E)
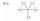

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 95 في هذه المجموعة.
فتح الحزمة
k this deck
50
In the best Lewis structure for the fulminate ion,CNO-,what is the formal charge on the central nitrogen atom?
A)+2
B)+1
C)0
D)-1
E)-2
A)+2
B)+1
C)0
D)-1
E)-2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 95 في هذه المجموعة.
فتح الحزمة
k this deck
51
In which one of the following is the best Lewis structure a resonance structure?
A)SO3
B)BF3
C)I3-
D)SCO (C = central atom)
E)SO32-
A)SO3
B)BF3
C)I3-
D)SCO (C = central atom)
E)SO32-

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 95 في هذه المجموعة.
فتح الحزمة
k this deck
52
In which one of the following structures does the central atom have a formal charge of +2?
A)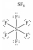
B)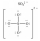
C)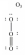
D)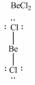
E)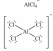
A)

B)
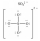
C)
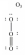
D)
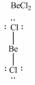
E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 95 في هذه المجموعة.
فتح الحزمة
k this deck
53
Which of these choices is the best Lewis structure for ozone,O3?
A)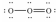
B)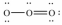
C)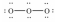
D)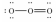
E)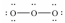
A)

B)
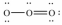
C)

D)
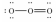
E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 95 في هذه المجموعة.
فتح الحزمة
k this deck
54
What is the formal charge on phosphorus in a Lewis structure for the phosphate ion that satisfies the octet rule?
A)-2
B)-1
C)0
D)+1
E)+2
A)-2
B)-1
C)0
D)+1
E)+2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 95 في هذه المجموعة.
فتح الحزمة
k this deck
55
In which one of the following species is the central atom (the first atom in the formula)likely to violate the octet rule?
A)BF4-
B)XeO3
C)SiCl4
D)NH3
E)CH2Cl2
A)BF4-
B)XeO3
C)SiCl4
D)NH3
E)CH2Cl2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 95 في هذه المجموعة.
فتح الحزمة
k this deck
56
The number of resonance structures for the sulfur dioxide molecule that satisfy the octet rule is
A)1.
B)2.
C)3.
D)4.
E)none of these
A)1.
B)2.
C)3.
D)4.
E)none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 95 في هذه المجموعة.
فتح الحزمة
k this deck
57
Hydrazine,N2H4,is a good reducing agent that has been used as a component in rocket fuels. Select its Lewis structure.
A)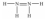
B)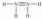
C)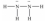
D)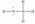
E)None of the choices is correct.
A)
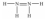
B)
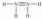
C)
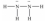
D)
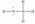
E)None of the choices is correct.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 95 في هذه المجموعة.
فتح الحزمة
k this deck
58
What is wrong with this Lewis structure? 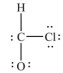
A)There are too many electrons.
B)There are too few electrons.
C)The O atom does not have an octet.
D)The C atom does not have an octet.
E)There is nothing wrong with this Lewis structure.

A)There are too many electrons.
B)There are too few electrons.
C)The O atom does not have an octet.
D)The C atom does not have an octet.
E)There is nothing wrong with this Lewis structure.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 95 في هذه المجموعة.
فتح الحزمة
k this deck
59
Select the Lewis structure for XeO2F2 which correctly minimizes formal charges.
A)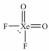
B)
C)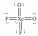
D)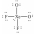
E)
A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 95 في هذه المجموعة.
فتح الحزمة
k this deck
60
Which of these substances will display an incomplete octet in its Lewis structure?
A)CO2
B)Cl2
C)ICl
D)NO
E)SO2
A)CO2
B)Cl2
C)ICl
D)NO
E)SO2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 95 في هذه المجموعة.
فتح الحزمة
k this deck
61
In Ba(CN)2,the bonding is
A)essentially ionic.
B)essentially covalent.
C)both ionic and covalent.
D)neither ionic nor covalent.
A)essentially ionic.
B)essentially covalent.
C)both ionic and covalent.
D)neither ionic nor covalent.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 95 في هذه المجموعة.
فتح الحزمة
k this deck
62
Ignoring resonance,the formal charge on the nitrogen atom in the nitrate ion is
A)+2.
B)+1.
C)0.
D)-1.
E)-2.
A)+2.
B)+1.
C)0.
D)-1.
E)-2.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 95 في هذه المجموعة.
فتح الحزمة
k this deck
63
Ionic compounds may carry a net positive or net negative charge.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 95 في هذه المجموعة.
فتح الحزمة
k this deck
64
Based on ionic radii and charges,which of the following solids would have the greatest lattice energy?
A)SrO
B)CsI
C)MgCl2
D)KF
E)NaF
A)SrO
B)CsI
C)MgCl2
D)KF
E)NaF

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 95 في هذه المجموعة.
فتح الحزمة
k this deck
65
How many single bonds are bonded to sulfur in SF6?
A)2
B)4
C)6
D)7
E)8
A)2
B)4
C)6
D)7
E)8

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 95 في هذه المجموعة.
فتح الحزمة
k this deck
66
Which one of the following compounds is most likely to be ionic?
A)HNF2
B)H2CO
C)N2H4
D)CaCl2
E)ICl
A)HNF2
B)H2CO
C)N2H4
D)CaCl2
E)ICl

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 95 في هذه المجموعة.
فتح الحزمة
k this deck
67
What formal charge resides on the carbon of CO32-?
A)0
B)+1
C)+2
D)+3
E)+4
A)0
B)+1
C)+2
D)+3
E)+4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 95 في هذه المجموعة.
فتح الحزمة
k this deck
68
Which molecule has a Lewis structure that does not obey the octet rule?
A)N2O
B)CS2
C)PH3
D)CCl4
E)NO2
A)N2O
B)CS2
C)PH3
D)CCl4
E)NO2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 95 في هذه المجموعة.
فتح الحزمة
k this deck
69
Which of the following represents the most polar bond?
A)B-C
B)S-O
C)C-O
D)B-O
E)C-C
A)B-C
B)S-O
C)C-O
D)B-O
E)C-C

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 95 في هذه المجموعة.
فتح الحزمة
k this deck
70
How many total resonance structures can be drawn for the sulfate ion,SO42-?
A)2
B)3
C)4
D)5
E)6
A)2
B)3
C)4
D)5
E)6

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 95 في هذه المجموعة.
فتح الحزمة
k this deck
71
Identify the correct equation to calculate the formal charge on an atom.
A)formal charge = valence electrons - lone pair electrons
B)formal charge = valence electrons - associated electrons
C)formal charge = valence electrons - bonding electrons
D)formal charge = bonding electrons + lone pair electrons
E)formal charge = valence electrons - bonding electrons + lone pair electrons
A)formal charge = valence electrons - lone pair electrons
B)formal charge = valence electrons - associated electrons
C)formal charge = valence electrons - bonding electrons
D)formal charge = bonding electrons + lone pair electrons
E)formal charge = valence electrons - bonding electrons + lone pair electrons

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 95 في هذه المجموعة.
فتح الحزمة
k this deck
72
In which of the following species does the central atom violate the octet rule?
A)CH4
B)SF4
C)PCl4+
D)CCl3+
E)NH3
A)CH4
B)SF4
C)PCl4+
D)CCl3+
E)NH3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 95 في هذه المجموعة.
فتح الحزمة
k this deck
73
Which response includes all the molecules below that have a central atom that does not follow the octet rule? (1)H2S,(2)BCl3,(3)PH3,(4)SF4
A)(2)and (4)
B)(2)and (3)
C)(1)and (2)
D)(3)and (4)
E)(1)and (4)
A)(2)and (4)
B)(2)and (3)
C)(1)and (2)
D)(3)and (4)
E)(1)and (4)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 95 في هذه المجموعة.
فتح الحزمة
k this deck
74
Ionic compounds tend to form between metals and nonmetals when electrons are transferred from an element with high ionization energy (metal)to an element with a low electron affinity (nonmetal).

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 95 في هذه المجموعة.
فتح الحزمة
k this deck
75
Which of these molecules has an atom with an expanded octet?
A)HCl
B)AsCl5
C)ICl
D)NCl3
E)Cl2
A)HCl
B)AsCl5
C)ICl
D)NCl3
E)Cl2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 95 في هذه المجموعة.
فتح الحزمة
k this deck
76
Which of these elements is most likely to exhibit an expanded octet in its compounds?
A)O
B)S
C)Na
D)C
E)N
A)O
B)S
C)Na
D)C
E)N

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 95 في هذه المجموعة.
فتح الحزمة
k this deck
77
If an element is bonded to 4 other atoms and has a formal charge of +1,what group must the element be in?
A)3A
B)4A
C)5A
D)6A
E)7A
A)3A
B)4A
C)5A
D)6A
E)7A

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 95 في هذه المجموعة.
فتح الحزمة
k this deck
78
All of the following molecules contain only single bonds except
A)H2O2.
B)Cl2.
C)CH4.
D)H2O.
E)SO2.
A)H2O2.
B)Cl2.
C)CH4.
D)H2O.
E)SO2.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 95 في هذه المجموعة.
فتح الحزمة
k this deck
79
What is the formal charge on the fluorine in ClF2+?
A)-1
B)+1
C)0
D)-2
E)-3
A)-1
B)+1
C)0
D)-2
E)-3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 95 في هذه المجموعة.
فتح الحزمة
k this deck
80
Which statement is false concerning ionic bonds and compounds?
A)Ionic bonds are a result of electrostatic forces.
B)Ionic bonds usually occur between elements with high and low electron affinities.
C)Elements of an ionic compound usually carry the same charge.
D)Lewis dot symbols are useful for tracking electrons.
A)Ionic bonds are a result of electrostatic forces.
B)Ionic bonds usually occur between elements with high and low electron affinities.
C)Elements of an ionic compound usually carry the same charge.
D)Lewis dot symbols are useful for tracking electrons.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 95 في هذه المجموعة.
فتح الحزمة
k this deck








