Deck 1: Structure and Bonding
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/127
العب
ملء الشاشة (f)
Deck 1: Structure and Bonding
1
The formal charge on the nitrogen on the structure shown below is: 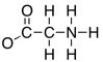

N = +1
2
Provide the electron configuration of phosphorus.
1s22s22p63s23p3
3
Draw the Lewis structure for 2-propanol, CH3CH(OH)CH3, including all non-bonding lone pairs.
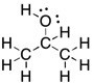
4
The electron density of ________ orbitals has spherical symmetry.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 127 في هذه المجموعة.
فتح الحزمة
k this deck
5
How many distinct p orbitals exist in the second electron shell, where n = 2?
A) 2
B) 3
C) 4
D) 5
E) 6
A) 2
B) 3
C) 4
D) 5
E) 6

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 127 في هذه المجموعة.
فتح الحزمة
k this deck
6
A node is a region of high electron density between the two atoms in a covalent bond.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 127 في هذه المجموعة.
فتح الحزمة
k this deck
7
Draw the shape of a 2p orbital, including shading to indicate phase.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 127 في هذه المجموعة.
فتح الحزمة
k this deck
8
Which element in the second row of the periodic table has six valence electrons and a valence of two?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 127 في هذه المجموعة.
فتح الحزمة
k this deck
9
Provide a Lewis structure for a molecule with molecular formula CH2O2.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 127 في هذه المجموعة.
فتح الحزمة
k this deck
10
In a carbon atom, the 2s and 2p orbitals are the same energy.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 127 في هذه المجموعة.
فتح الحزمة
k this deck
11
Atoms with the same number of protons but different numbers of neutrons are called ________.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 127 في هذه المجموعة.
فتح الحزمة
k this deck
12
Draw the Lewis structure of acetic acid, CH3CO2H, including all non-bonding lone pairs.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 127 في هذه المجموعة.
فتح الحزمة
k this deck
13
The ________ tells us that each orbital can hold a maximum of 2 electrons.
A) aufbau principle
B) Pauli exclusion principle
C) Hund's rule principle
D) LeChatelier principle
E) uncertainty principle
A) aufbau principle
B) Pauli exclusion principle
C) Hund's rule principle
D) LeChatelier principle
E) uncertainty principle

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 127 في هذه المجموعة.
فتح الحزمة
k this deck
14
The atomic number of boron is 5. The correct electronic configuration of boron is:
A) 1s22s3
B) 1s22p3
C) 1s22s22p1
D) 2s22p3
E) 1s22s23s1
A) 1s22s3
B) 1s22p3
C) 1s22s22p1
D) 2s22p3
E) 1s22s23s1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 127 في هذه المجموعة.
فتح الحزمة
k this deck
15
While you were up late one night studying organic chemistry, you happened to see the last 5 minutes of an infomercial on TV. The spokesperson claimed that their brand of automobile tires were superior to all other brands on the market because they were made by using only natural rubber, isolated from the resin of rubber trees. How could a chemist test her claims that no petroleum products went into the manufacture of her brand of tires?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 127 في هذه المجموعة.
فتح الحزمة
k this deck
16
An oxygen atom has ________ valence electrons.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 127 في هذه المجموعة.
فتح الحزمة
k this deck
17
Orbitals which are equal in energy are referred to as ________.
A) degenerate
B) polar
C) nodes
D) filled
E) nonpolar
A) degenerate
B) polar
C) nodes
D) filled
E) nonpolar

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 127 في هذه المجموعة.
فتح الحزمة
k this deck
18
When filling two or more orbitals of the same energy with electrons, the electrons will go into different orbitals rather than pair up in the same orbital.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 127 في هذه المجموعة.
فتح الحزمة
k this deck
19
Draw a correct Lewis structure for chloromethane, CH3Cl, including all non-bonding lone pairs.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 127 في هذه المجموعة.
فتح الحزمة
k this deck
20
The element with the electronic configuration 1s22s22p63s1 is ________.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 127 في هذه المجموعة.
فتح الحزمة
k this deck
21
Add the appropriate formal charge to each atom in the molecule below. It is not necessary to indicate formal charges when zero. (All non-bonding electrons are included.) 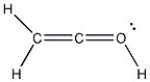
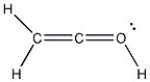

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 127 في هذه المجموعة.
فتح الحزمة
k this deck
22
The formal charge on the oxygens in the compound below are ________. 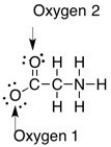
A) Oxygen 1: 0, Oxygen 2: 0
B) Oxygen 1: -1, Oxygen 2: 0
C) Oxygen 1: 0, Oxygen 2: -1
D) Oxygen 1: +1, Oxygen 2: 0
E) Oxygen 1: -1, Oxygen 2: -1

A) Oxygen 1: 0, Oxygen 2: 0
B) Oxygen 1: -1, Oxygen 2: 0
C) Oxygen 1: 0, Oxygen 2: -1
D) Oxygen 1: +1, Oxygen 2: 0
E) Oxygen 1: -1, Oxygen 2: -1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 127 في هذه المجموعة.
فتح الحزمة
k this deck
23
Add the appropriate formal charge to each atom in the molecule below. It is not necessary to indicate formal charges when zero. (All non-bonding electrons are included.) 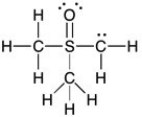


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 127 في هذه المجموعة.
فتح الحزمة
k this deck
24
One or more of the atoms in the structure shown should have nonzero formal charges. Add the correct formal charge/s. (All non-bonding electrons have been included.) 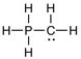


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 127 في هذه المجموعة.
فتح الحزمة
k this deck
25
The formal charge on oxygen in dimethyl ether, CH3OCH3, is ________.
A) +2
B) +1
C) 0
D) -1
E) -2
A) +2
B) +1
C) 0
D) -1
E) -2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 127 في هذه المجموعة.
فتح الحزمة
k this deck
26
Draw a correct Lewis structure for acetonitrile, CH3CN, including all non-bonding lone pairs.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 127 في هذه المجموعة.
فتح الحزمة
k this deck
27
Draw 2 possible Lewis structures for the compound with molecular formula C3H6.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 127 في هذه المجموعة.
فتح الحزمة
k this deck
28
For most compounds in which a nitrogen atom bears no formal charge, the valence of this nitrogen atom is ________.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 127 في هذه المجموعة.
فتح الحزمة
k this deck
29
Assign the correct formal charge to each nitrogen atom in the following Lewis structure. (All non-bonding electrons are included.) 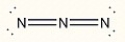
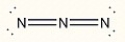

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 127 في هذه المجموعة.
فتح الحزمة
k this deck
30
One or more of the atoms in the structure shown should have nonzero formal charges. Redraw the structure and the atoms with non-zero formal charges. 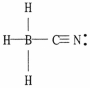


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 127 في هذه المجموعة.
فتح الحزمة
k this deck
31
The electronegativity of elements on the periodic table increases going ________ a column and to the ________ in each row.
A) up; right
B) up; left
C) down; right
D) down; left
A) up; right
B) up; left
C) down; right
D) down; left

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 127 في هذه المجموعة.
فتح الحزمة
k this deck
32
Which of the following molecules contains a polar covalent bond?
A) H2
B) F2
C) CH3Cl
D) NaCl
E) He
A) H2
B) F2
C) CH3Cl
D) NaCl
E) He

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 127 في هذه المجموعة.
فتح الحزمة
k this deck
33
A carbon-hydrogen bond in ethane (CH3CH3) is best described a ________.
A) highly polar
B) essentially nonpolar
C) ionic
D) a multiple bond
E) resonance stabilized
A) highly polar
B) essentially nonpolar
C) ionic
D) a multiple bond
E) resonance stabilized

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 127 في هذه المجموعة.
فتح الحزمة
k this deck
34
Which of the following are acceptable Lewis structures, including formal charges, for nitric acid, HNO3? 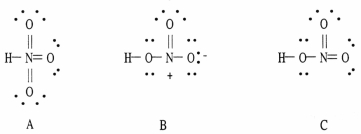
A) A only
B) B only
C) C only
D) both B and C
E) A, B, and C

A) A only
B) B only
C) C only
D) both B and C
E) A, B, and C

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 127 في هذه المجموعة.
فتح الحزمة
k this deck
35
Draw a correct Lewis structure for tert-butyl alcohol, (CH3)3COH, including all non-bonding lone pairs.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 127 في هذه المجموعة.
فتح الحزمة
k this deck
36
Covalent bonds may be polar or nonpolar. What property of the atoms forming a given bond determines this?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 127 في هذه المجموعة.
فتح الحزمة
k this deck
37
Draw the Lewis structure for boric acid, B(OH)3, including all non-bonding lone pairs.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 127 في هذه المجموعة.
فتح الحزمة
k this deck
38
Within a given row of the periodic table, electronegativity typically increases left to right across the row.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 127 في هذه المجموعة.
فتح الحزمة
k this deck
39
The compound methylamine, CH3NH2, contains a C-N bond. In this bond, which of the following best describes the charge on the carbon atom?
A) +1
B) slightly positive
C) neutral
D) slightly negative
E) -1
A) +1
B) slightly positive
C) neutral
D) slightly negative
E) -1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 127 في هذه المجموعة.
فتح الحزمة
k this deck
40
Write a Lewis structure for a compound with the molecular formula H2N2.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 127 في هذه المجموعة.
فتح الحزمة
k this deck
41
The Lewis structure of trimethylamine is shown below. Draw the condensed structural formula which corresponds to this Lewis structure. 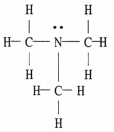


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 127 في هذه المجموعة.
فتح الحزمة
k this deck
42
Nitroamines are common functional groups found in energetic materials, such as RDX and HMX. For the structure below, draw two other significant resonance structures, include any formal charges, and indicate the hybridization on each nitrogen and oxygen. 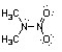
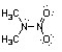

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 127 في هذه المجموعة.
فتح الحزمة
k this deck
43
Which of the following compounds are covalent compounds?
A) KCl
B) CF4
C) NH3
D) both A and B
E) both B and C
A) KCl
B) CF4
C) NH3
D) both A and B
E) both B and C

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 127 في هذه المجموعة.
فتح الحزمة
k this deck
44
When a negatively charged species is most appropriately depicted as a hybrid of several resonance forms, the negative charge present is considered to be rapidly moving between the resonance forms bearing the formal negative charge.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 127 في هذه المجموعة.
فتح الحزمة
k this deck
45
Structures ________, shown below, are resonance structures, and structure ________ is the major contributor to the overall resonance hybrid. 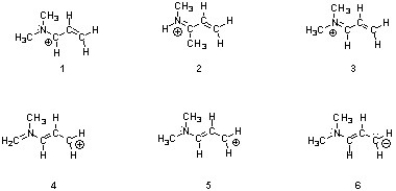
A) 2 & 4; 2
B) 1, 3 & 5; 3
C) 4 & 6; 6
D) 1, 3 & 5; 1
E) 1, 3, 4 & 5; 3

A) 2 & 4; 2
B) 1, 3 & 5; 3
C) 4 & 6; 6
D) 1, 3 & 5; 1
E) 1, 3, 4 & 5; 3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 127 في هذه المجموعة.
فتح الحزمة
k this deck
46
Draw the important resonance forms for the structure shown below. 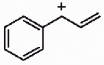


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 127 في هذه المجموعة.
فتح الحزمة
k this deck
47
In the compound sodium methoxide (NaOCH3), there is ________ bonding.
A) ionic
B) polar covalent
C) nonpolar covalent
D) a mixture of ionic and covalent
E) resonance stabilized
A) ionic
B) polar covalent
C) nonpolar covalent
D) a mixture of ionic and covalent
E) resonance stabilized

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 127 في هذه المجموعة.
فتح الحزمة
k this deck
48
Draw the complete Lewis structure for the compound whose condensed formula is (CH3)2CHCHO.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 127 في هذه المجموعة.
فتح الحزمة
k this deck
49
One resonance structure of a cation is shown. Provide the other reasonable resonance structures. 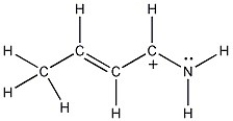


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 127 في هذه المجموعة.
فتح الحزمة
k this deck
50
Draw the other important resonance form of: 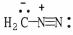
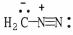

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 127 في هذه المجموعة.
فتح الحزمة
k this deck
51
Draw a line-angle formula for (CH3)2CHCH2CH2NH2.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 127 في هذه المجموعة.
فتح الحزمة
k this deck
52
Which of the following structures (a-d) is another resonance structure of the following organic molecule? 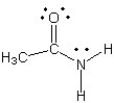
A)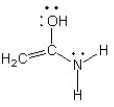
B)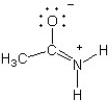
C)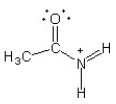
D)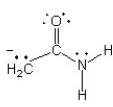

A)

B)

C)

D)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 127 في هذه المجموعة.
فتح الحزمة
k this deck
53
When a molecule can best be represented as a series of resonance forms, each of these forms always contributes to the same degree in the hybrid.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 127 في هذه المجموعة.
فتح الحزمة
k this deck
54
The Lewis structure of pentane is shown below. Draw the condensed structural formula which corresponds to this Lewis structure. 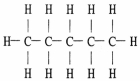
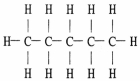

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 127 في هذه المجموعة.
فتح الحزمة
k this deck
55
Draw 3 significant resonance structures for the compound shown below. Place a box around the major contributor. Fill in any missing formal charges. 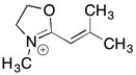


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 127 في هذه المجموعة.
فتح الحزمة
k this deck
56
Draw additional resonance contributors for: 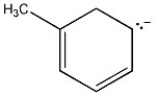


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 127 في هذه المجموعة.
فتح الحزمة
k this deck
57
Which of the following bonding patterns of carbon is not allowed in the formation of an organic compound?
A)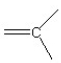
B)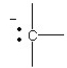
C)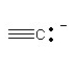
D)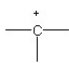
E)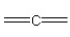
F)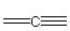
A)

B)

C)
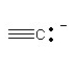
D)

E)
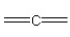
F)
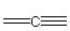

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 127 في هذه المجموعة.
فتح الحزمة
k this deck
58
Draw the important resonance forms for the structure shown below. 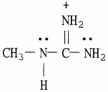


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 127 في هذه المجموعة.
فتح الحزمة
k this deck
59
Which of the following choices represent(s) a pair of resonance forms?
A)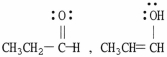
B)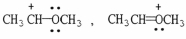
C)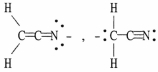
D) both A and C
E) both B and C
A)
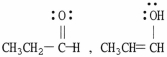
B)
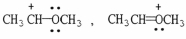
C)
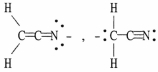
D) both A and C
E) both B and C

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 127 في هذه المجموعة.
فتح الحزمة
k this deck
60
Draw the important resonance forms of the structure below to indicate the delocalization of charge. Indicate which is the major contributor to the overall structure. 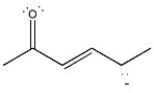


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 127 في هذه المجموعة.
فتح الحزمة
k this deck
61
Draw the line-angle formula for three compounds with molecular formula C3H8O.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 127 في هذه المجموعة.
فتح الحزمة
k this deck
62
What is the molecular formula for the following line-angle structure? 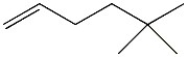


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 127 في هذه المجموعة.
فتح الحزمة
k this deck
63
Which of the following condensed formulas correctly represents the line-angle structure shown below? 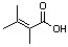
A) CH(CH3)2CH(CH3)CO2H
B) C2(CH3)3CO2H
C) (CH3)2CC(CH3)CO2H
D) C(CH3)2C(CH3)CH2CO2H

A) CH(CH3)2CH(CH3)CO2H
B) C2(CH3)3CO2H
C) (CH3)2CC(CH3)CO2H
D) C(CH3)2C(CH3)CH2CO2H

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 127 في هذه المجموعة.
فتح الحزمة
k this deck
64
Provide the line-angle formula (skeletal structure) for (CH3)2CHCH2CHO.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 127 في هذه المجموعة.
فتح الحزمة
k this deck
65
Which of the following condensed formulas represents the same compound as the line-angle structure shown? 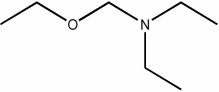
A) CH3CH2CH2OCH2CH2CH2N(CH2CH2CH3)2
B) CH3CH2CH2OCH2N(CH2CH3)2
C) CH3CH2OCH2N(CH2CH3)2
D) CH3CH2OCH2N(CH2CH2CH3)2
E) CH3ON(CH3)2

A) CH3CH2CH2OCH2CH2CH2N(CH2CH2CH3)2
B) CH3CH2CH2OCH2N(CH2CH3)2
C) CH3CH2OCH2N(CH2CH3)2
D) CH3CH2OCH2N(CH2CH2CH3)2
E) CH3ON(CH3)2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 127 في هذه المجموعة.
فتح الحزمة
k this deck
66
How many carbon atoms are present in the molecule shown? 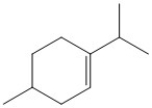
A) 6
B) 8
C) 10
D) 11
E) 12

A) 6
B) 8
C) 10
D) 11
E) 12

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 127 في هذه المجموعة.
فتح الحزمة
k this deck
67
A condensed structure for acetone is CH3COCH3. Provide the structural formula for acetone.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 127 في هذه المجموعة.
فتح الحزمة
k this deck
68
Draw a correct Lewis structure for CH3CHCHCOOH.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 127 في هذه المجموعة.
فتح الحزمة
k this deck
69
Draw an acceptable line-angle formula for the compound shown below. 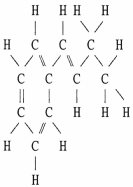


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 127 في هذه المجموعة.
فتح الحزمة
k this deck
70
Provide the line-angle formula (skeletal structure) for (CH3CH2)2C=O.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 127 في هذه المجموعة.
فتح الحزمة
k this deck
71
How many hydrogen atoms are present in the molecule shown? 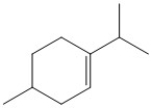


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 127 في هذه المجموعة.
فتح الحزمة
k this deck
72
Draw a correct Lewis structure for acetaldehyde, CH3CHO.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 127 في هذه المجموعة.
فتح الحزمة
k this deck
73
Compute the empirical and molecular formulas for the compound of molecular weight 180 g/mol which is shown to contain 40.0% C and 6.7% H by elemental analysis.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 127 في هذه المجموعة.
فتح الحزمة
k this deck
74
Provide the line-angle formula for the alcohol CH3CH2CH(OH)CH2CH2CH(CH3)2.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 127 في هذه المجموعة.
فتح الحزمة
k this deck
75
Provide the line-angle formula for CH3CH2C(CH3)2CH2CHO

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 127 في هذه المجموعة.
فتح الحزمة
k this deck
76
Draw an acceptable line-angle formula for cyclobutanol (shown below). 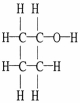


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 127 في هذه المجموعة.
فتح الحزمة
k this deck
77
What is the molecular formula for the following line-angle structure? 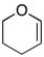


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 127 في هذه المجموعة.
فتح الحزمة
k this deck
78
Draw a complete Lewis structure, including lone pairs, for (CH3)2CHCO2H.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 127 في هذه المجموعة.
فتح الحزمة
k this deck
79
Draw condensed structures for the four compounds with formula C3H9N.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 127 في هذه المجموعة.
فتح الحزمة
k this deck
80
Indicate the line-angle structure that corresponds to the condensed structure, HOCH2C(O)CH(CH3)2.
A)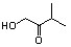
B)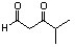
C)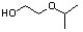
D)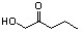
A)

B)

C)

D)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 127 في هذه المجموعة.
فتح الحزمة
k this deck








