Deck 4: The Study of Chemical Reactions
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/128
العب
ملء الشاشة (f)
Deck 4: The Study of Chemical Reactions
1
Write a detailed, stepwise mechanism for the following reaction. 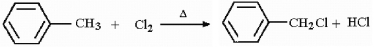

2
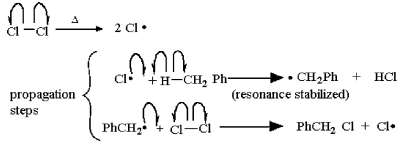
Which of the following is a propagation step in the free radical chlorination of dichloromethane?
A) ∙ CHCl2 + Cl2 → CHCl3 + Cl∙
B) ∙ CHCl2 + Cl∙ → CHCl3
C) CH2Cl2 + Cl∙ → CHCl3 + H∙
D) Cl2 + UV light → 2 Cl∙
E) ∙ CHCl2 + ∙ CHCl2 → CHCl2CHCl2
A) ∙ CHCl2 + Cl2 → CHCl3 + Cl∙
B) ∙ CHCl2 + Cl∙ → CHCl3
C) CH2Cl2 + Cl∙ → CHCl3 + H∙
D) Cl2 + UV light → 2 Cl∙
E) ∙ CHCl2 + ∙ CHCl2 → CHCl2CHCl2
∙ CHCl2 + Cl2 → CHCl3 + Cl∙
3
Write an equation to describe the initiation step in the chlorination of methane.
Cl-Cl + photon (hν) → 2 Cl∙
4
When the reaction between methane and chlorine is photochemically initiated, which of the following compounds cannot be formed through a termination reaction?
A) CH3Cl
B) HCl
C) CH3CH3
D) Cl2
A) CH3Cl
B) HCl
C) CH3CH3
D) Cl2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 128 في هذه المجموعة.
فتح الحزمة
k this deck
5
What is meant by the mechanism of a chemical reaction?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 128 في هذه المجموعة.
فتح الحزمة
k this deck
6
When light is shined on a mixture of chlorine and chloromethane, carbon tetrachloride is one of the components of the final reaction mixture. Propose a series of mechanistic steps which explain this observation.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 128 في هذه المجموعة.
فتح الحزمة
k this deck
7
Draw the product of the monochlorination of methane with Cl2 in the presence of light or heat.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 128 في هذه المجموعة.
فتح الحزمة
k this deck
8
Chlorination of methane can result in a mixture of chlorinated products. What experimental conditions should be used to favor the production of chloromethane over the other chlorinated products?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 128 في هذه المجموعة.
فتح الحزمة
k this deck
9
Which of the following is not a possible termination step in the free radical chlorination of methane?
A) ∙CH3 + Cl2 → CH3Cl + Cl∙
B) ∙CH3 + Cl∙ → CH3Cl
C) ∙CH3 + ∙CH3 → CH3CH3
D) ∙CH3 + wall → CH3-wall
E) Cl∙ + wall → Cl-wall
A) ∙CH3 + Cl2 → CH3Cl + Cl∙
B) ∙CH3 + Cl∙ → CH3Cl
C) ∙CH3 + ∙CH3 → CH3CH3
D) ∙CH3 + wall → CH3-wall
E) Cl∙ + wall → Cl-wall

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 128 في هذه المجموعة.
فتح الحزمة
k this deck
10
Which of the following species is not formed through a termination reaction in the chlorination of methane?
A) CH3Cl
B) HCl
C) H2
D) CH3CH3
A) CH3Cl
B) HCl
C) H2
D) CH3CH3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 128 في هذه المجموعة.
فتح الحزمة
k this deck
11
In the reaction of Cl2 with ethane and UV light, which of the following reactions would be a propagation event(s)?
I. Cl∙ + CH3-CH3 → CH3-CH2-Cl + H∙
II. Cl∙ + CH3-CH3 → CH3-H2C∙ + HCl
III. Cl∙ + CH3-H2C∙ → CH3-CH2-Cl
IV. Cl2 + CH3-H2C∙ → CH3-CH2-Cl + Cl∙
V. Cl2 + UV light → C l∙ + Cl∙
A) reactions I and V
B) reactions II, III and IV
C) reactions I and IV
D) reactions II and IV
E) reactions I, II and IV
I. Cl∙ + CH3-CH3 → CH3-CH2-Cl + H∙
II. Cl∙ + CH3-CH3 → CH3-H2C∙ + HCl
III. Cl∙ + CH3-H2C∙ → CH3-CH2-Cl
IV. Cl2 + CH3-H2C∙ → CH3-CH2-Cl + Cl∙
V. Cl2 + UV light → C l∙ + Cl∙
A) reactions I and V
B) reactions II, III and IV
C) reactions I and IV
D) reactions II and IV
E) reactions I, II and IV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 128 في هذه المجموعة.
فتح الحزمة
k this deck
12
In the reaction of Cl2 with ethane and UV light, which of the following reactions would be a chain termination event(s)?
I. Cl∙ + CH3-CH3 → CH3-CH2-Cl + H∙
II. Cl∙ + CH3-CH3 → CH3-H2C∙ + HCl
III. Cl∙ + CH3-H2C∙ → CH3-CH2-Cl
IV. Cl2 + CH3-H2C∙ → CH3-CH2-Cl + Cl∙
V. Cl2 + UV light → C l∙ + Cl∙
A) reaction V
B) reactions I and IV
C) reactions III and IV
D) reactions I and II
E) reaction III
I. Cl∙ + CH3-CH3 → CH3-CH2-Cl + H∙
II. Cl∙ + CH3-CH3 → CH3-H2C∙ + HCl
III. Cl∙ + CH3-H2C∙ → CH3-CH2-Cl
IV. Cl2 + CH3-H2C∙ → CH3-CH2-Cl + Cl∙
V. Cl2 + UV light → C l∙ + Cl∙
A) reaction V
B) reactions I and IV
C) reactions III and IV
D) reactions I and II
E) reaction III

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 128 في هذه المجموعة.
فتح الحزمة
k this deck
13
________ is the study of reaction rates.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 128 في هذه المجموعة.
فتح الحزمة
k this deck
14
In the first propagation step of the free radical chlorination of methane, which of the following occurs?
A) Cl2 dissociates.
B) A chlorine radical abstracts a hydrogen.
C) A carbon radical reacts with Cl2.
D) A carbon radical reacts with a chlorine radical.
E) Two chlorine radicals combine.
A) Cl2 dissociates.
B) A chlorine radical abstracts a hydrogen.
C) A carbon radical reacts with Cl2.
D) A carbon radical reacts with a chlorine radical.
E) Two chlorine radicals combine.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 128 في هذه المجموعة.
فتح الحزمة
k this deck
15
Provide the two propagation steps in the free-radical chlorination of ethane.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 128 في هذه المجموعة.
فتح الحزمة
k this deck
16
Draw the important resonance form/s of the following free radical. 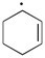


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 128 في هذه المجموعة.
فتح الحزمة
k this deck
17
The monochlorination of butane with chlorine gas under photolysis will give two products. Draw both of them and then predict the product ratio.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 128 في هذه المجموعة.
فتح الحزمة
k this deck
18
Species with unpaired electrons are called ________.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 128 في هذه المجموعة.
فتح الحزمة
k this deck
19
In the presence of a small amount of bromine, the following light-promoted reaction has been observed. Write a mechanism for this reaction, making sure to explain how both products are formed. 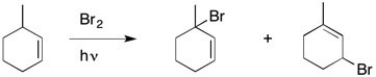


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 128 في هذه المجموعة.
فتح الحزمة
k this deck
20
For the compound below, the number of 1°, 2° and 3° hydrogens, respectively is ________. 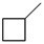
A) 1, 3, and 1
B) 3, 6 and 2
C) 3, 6 and 1
D) 1, 6 and 0

A) 1, 3, and 1
B) 3, 6 and 2
C) 3, 6 and 1
D) 1, 6 and 0

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 128 في هذه المجموعة.
فتح الحزمة
k this deck
21
For the reaction A + B → C + D, ΔG° = -5.00 kcal/mol. What is the corresponding equilibrium constant at 
R = 1.987 cal/mol∙K.

R = 1.987 cal/mol∙K.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 128 في هذه المجموعة.
فتح الحزمة
k this deck
22
Given a ΔG° of -8.0 kJ/mol at 25°C, calculate the corresponding K. [R = 8.314 J/K∙ mol]

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 128 في هذه المجموعة.
فتح الحزمة
k this deck
23
Given a K of 0.45 at 25°C, calculate the corresponding ΔG° in kJ/mol. [R = 8.314 J/K∙ mol]

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 128 في هذه المجموعة.
فتح الحزمة
k this deck
24
Given a K of 2.2 at 25°C, calculate the corresponding ΔG° in kJ/mol. [R = 8.314 J/K∙ mol]

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 128 في هذه المجموعة.
فتح الحزمة
k this deck
25
Which is a measure of the randomness of a system?
A) entropy
B) enthalpy
C) free energy
D) halogenation
E) stoichiometry
A) entropy
B) enthalpy
C) free energy
D) halogenation
E) stoichiometry

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 128 في هذه المجموعة.
فتح الحزمة
k this deck
26
If the percentage of the axial onformer (on the product side of the reaction) is 42%, what is the ΔG° (in kJ/mol) for the reaction at 25°C? [R = 8.314 J/K∙ mol] ![If the percentage of the axial onformer (on the product side of the reaction) is 42%, what is the ΔG° (in kJ/mol) for the reaction at 25°C? [R = 8.314 J/K∙ mol]](https://d2lvgg3v3hfg70.cloudfront.net/TB6199/11eab5e1_c8c5_da05_9bae_c18871bd2550_TB6199_00.jpg)
![If the percentage of the axial onformer (on the product side of the reaction) is 42%, what is the ΔG° (in kJ/mol) for the reaction at 25°C? [R = 8.314 J/K∙ mol]](https://d2lvgg3v3hfg70.cloudfront.net/TB6199/11eab5e1_c8c5_da05_9bae_c18871bd2550_TB6199_00.jpg)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 128 في هذه المجموعة.
فتح الحزمة
k this deck
27
Consider the transformation of A to B (i.e., A →
B). If at equilibrium at 25°C the concentration of A is 20% of the initial concentration of A, determine the value of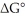
(in kcal/mol) for this reaction. R = 1.987 cal/mol∙K.
B). If at equilibrium at 25°C the concentration of A is 20% of the initial concentration of A, determine the value of
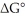
(in kcal/mol) for this reaction. R = 1.987 cal/mol∙K.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 128 في هذه المجموعة.
فتح الحزمة
k this deck
28
If a reaction is exothermic, then ________.
A) ΔS° < 0
B) ΔS° > 0
C) ΔH° < 0
D) ΔH° > 0
E) both B and D
A) ΔS° < 0
B) ΔS° > 0
C) ΔH° < 0
D) ΔH° > 0
E) both B and D

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 128 في هذه المجموعة.
فتح الحزمة
k this deck
29
Which of the following is true for the initiation step of a free radical chlorination reaction?
A) ΔH° > 0 and ΔS° > 0
B) ΔH° > 0 and ΔS° < 0
C) ΔH° < 0 and ΔS° > 0
D) ΔH° < 0 and ΔS° < 0
E) ΔH° = 0 and ΔS° = 0
A) ΔH° > 0 and ΔS° > 0
B) ΔH° > 0 and ΔS° < 0
C) ΔH° < 0 and ΔS° > 0
D) ΔH° < 0 and ΔS° < 0
E) ΔH° = 0 and ΔS° = 0

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 128 في هذه المجموعة.
فتح الحزمة
k this deck
30
Given a reaction in which reactant A is converted only to product B at 25°C, what Keq results if at equilibrium 80% of A has become B?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 128 في هذه المجموعة.
فتح الحزمة
k this deck
31
Which of the following statements correctly describes the contribution of 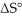 to
to 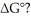
A) The entropy term makes a greater contribution to ΔG° at low temperatures.
B) The entropy term makes a greater contribution to ΔG° at high temperatures.
C) The entropy term makes a greater contribution to ΔG° in exothermic reactions.
D) The entropy term makes a greater contribution to ΔG° in endothermic reactions.
E) The entropy term always makes a more significant contribution to ΔG° than does the enthalpy term.
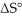 to
to 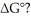
A) The entropy term makes a greater contribution to ΔG° at low temperatures.
B) The entropy term makes a greater contribution to ΔG° at high temperatures.
C) The entropy term makes a greater contribution to ΔG° in exothermic reactions.
D) The entropy term makes a greater contribution to ΔG° in endothermic reactions.
E) The entropy term always makes a more significant contribution to ΔG° than does the enthalpy term.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 128 في هذه المجموعة.
فتح الحزمة
k this deck
32
Assume the reaction A + B → C + D proceeds to equilibrium. Calculate the equilibrium concentration of D at  given that the starting concentrations of A and B are 2M and that △G° for the reaction is
given that the starting concentrations of A and B are 2M and that △G° for the reaction is 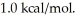
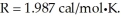
A) 0.40M
B) 0.60M
C) 1.00M
D) 1.40M
E) 1.60M
 given that the starting concentrations of A and B are 2M and that △G° for the reaction is
given that the starting concentrations of A and B are 2M and that △G° for the reaction is 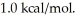
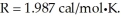
A) 0.40M
B) 0.60M
C) 1.00M
D) 1.40M
E) 1.60M

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 128 في هذه المجموعة.
فتح الحزمة
k this deck
33
Consider the reaction of A being converted into B at 25°C. If the ΔG° of this reaction is 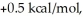 the Keq is ________ and the % conversion is ________.
the Keq is ________ and the % conversion is ________.
A) 0.18; 15%
B) 0.43; 30%
C) 1.0; 50%
D) 2.3; 70%
E) 5.4; 84%
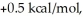 the Keq is ________ and the % conversion is ________.
the Keq is ________ and the % conversion is ________.A) 0.18; 15%
B) 0.43; 30%
C) 1.0; 50%
D) 2.3; 70%
E) 5.4; 84%

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 128 في هذه المجموعة.
فتح الحزمة
k this deck
34
Consider the following substitution reaction with a ΔG° value of -91.1 kJ/mole.
HO- + CH3Cl ↔ CH3OH + Cl-
Given this information which of the following statements must be true? (R = 8.315 J/mole K)
A) The Keq at 25°C for this reaction is very large, in other words this reaction proceeds to near completion as written, left to right under standard conditions.
B) The Keq at 25°C for this reaction is very small (<1), in other words this reaction does not proceed from left to right but rather is favored from right to left under standard conditions .
C) At 250°C the equilibrium concentration is shifted right in favor of the products (CH3OH and Cl-). In other words there is more product than at 25°C.
D) At 250°C the equilibrium concentration of products and reactants is nearly the same.
E) Both A and C are correct.
HO- + CH3Cl ↔ CH3OH + Cl-
Given this information which of the following statements must be true? (R = 8.315 J/mole K)
A) The Keq at 25°C for this reaction is very large, in other words this reaction proceeds to near completion as written, left to right under standard conditions.
B) The Keq at 25°C for this reaction is very small (<1), in other words this reaction does not proceed from left to right but rather is favored from right to left under standard conditions .
C) At 250°C the equilibrium concentration is shifted right in favor of the products (CH3OH and Cl-). In other words there is more product than at 25°C.
D) At 250°C the equilibrium concentration of products and reactants is nearly the same.
E) Both A and C are correct.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 128 في هذه المجموعة.
فتح الحزمة
k this deck
35
If the equilibrium constant (Keq) of a reaction is 0.5 then which of the following that must be true?
A) The reaction will have an early transition state.
B) Reaction equilibrium will favor the products.
C) Gibbs free energy (G) is positive.
D) Gibbs free energy (G) is negative.
A) The reaction will have an early transition state.
B) Reaction equilibrium will favor the products.
C) Gibbs free energy (G) is positive.
D) Gibbs free energy (G) is negative.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 128 في هذه المجموعة.
فتح الحزمة
k this deck
36
Given the pKa values for the two acids below, what would you expect the Keq for this reaction to be? 
A) Keq > 1
B) Keq = 1
C) Keq < 1
D) You can't tell from the information given.

A) Keq > 1
B) Keq = 1
C) Keq < 1
D) You can't tell from the information given.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 128 في هذه المجموعة.
فتح الحزمة
k this deck
37
Consider the reaction of A being converted into B at 25°C. If the ΔG° of this reaction is 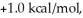 the Keq is ________ and the % conversion is ________.
the Keq is ________ and the % conversion is ________.
A) 0.18; 15%
B) 0.43; 30%
C) 1.0; 50%
D) 2.3; 70%
E) 5.4; 84%
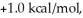 the Keq is ________ and the % conversion is ________.
the Keq is ________ and the % conversion is ________.A) 0.18; 15%
B) 0.43; 30%
C) 1.0; 50%
D) 2.3; 70%
E) 5.4; 84%

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 128 في هذه المجموعة.
فتح الحزمة
k this deck
38
In an exothermic reaction, are stronger bonds broken and weaker bonds formed or are weaker bonds broken and stronger bonds formed?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 128 في هذه المجموعة.
فتح الحزمة
k this deck
39
Which of the following correctly expresses the standard Gibbs free energy change of a reaction in terms of the changes in enthalpy and entropy?
A) ΔG° = ΔH° - TΔS°
B) ΔG° = ΔH° + TΔS°
C) ΔG° = ΔS° - TΔH°
D) ΔG° = ΔS° + TΔH°
E) none of the above
A) ΔG° = ΔH° - TΔS°
B) ΔG° = ΔH° + TΔS°
C) ΔG° = ΔS° - TΔH°
D) ΔG° = ΔS° + TΔH°
E) none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 128 في هذه المجموعة.
فتح الحزمة
k this deck
40
Consider the reaction of A being converted into B at 25°C. If the ΔG° of this reaction is 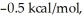 the Keq is ________ and the % conversion is ________.
the Keq is ________ and the % conversion is ________.
A) 0.18; 15%
B) 0.43; 30%
C) 1.0; 50%
D) 2.3; 70%
E) 5.4; 84%
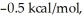 the Keq is ________ and the % conversion is ________.
the Keq is ________ and the % conversion is ________.A) 0.18; 15%
B) 0.43; 30%
C) 1.0; 50%
D) 2.3; 70%
E) 5.4; 84%

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 128 في هذه المجموعة.
فتح الحزمة
k this deck
41
Predict the enthalpy (ΔH) value for the theoretical reaction below, and indicate whether it is endothermic or exothermic. The bond dissociation energy for each bond in Kcal/mol is shown below each reactant and product.

A) +8 Kcal/mol, endothermic
B) -8 Kcal/mol, exothermic
C) +16 Kcal/mol, endothermic
D) +8 Kcal/mol, exothermic

A) +8 Kcal/mol, endothermic
B) -8 Kcal/mol, exothermic
C) +16 Kcal/mol, endothermic
D) +8 Kcal/mol, exothermic

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 128 في هذه المجموعة.
فتح الحزمة
k this deck
42
Which of the presented mechanisms would be the most energetically favorable and thus the most likely mechanism to actually occur for the following free radical chain reaction?
(bond dissociation energies - H-H = 104 kcal/mol; Cl-Cl = 58 kcal/mol; H-Cl = 103 kcal/mol)
H2 + Cl2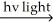 2 HCl
2 HCl
A) H2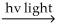 H∙ + H∙
H∙ + H∙
H∙ + Cl2 → Cl∙ + HCl
H∙ + Cl∙ → HCl
B) Cl2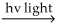 Cl∙ + Cl∙
Cl∙ + Cl∙
Cl∙ + H2 → H∙ + HCl
H∙ + Cl2 → HCl + Cl∙
C) H2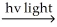 H∙ + H∙
H∙ + H∙
H∙ + Cl2 → Cl∙ + HCl
Cl∙ + H2 → HCl + H∙
D) Cl2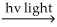 Cl∙ + Cl∙
Cl∙ + Cl∙
Cl∙ + H2 → H∙ + HCl
H∙ + Cl∙ → HCl
(bond dissociation energies - H-H = 104 kcal/mol; Cl-Cl = 58 kcal/mol; H-Cl = 103 kcal/mol)
H2 + Cl2
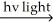 2 HCl
2 HClA) H2
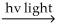 H∙ + H∙
H∙ + H∙H∙ + Cl2 → Cl∙ + HCl
H∙ + Cl∙ → HCl
B) Cl2
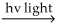 Cl∙ + Cl∙
Cl∙ + Cl∙Cl∙ + H2 → H∙ + HCl
H∙ + Cl2 → HCl + Cl∙
C) H2
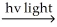 H∙ + H∙
H∙ + H∙H∙ + Cl2 → Cl∙ + HCl
Cl∙ + H2 → HCl + H∙
D) Cl2
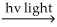 Cl∙ + Cl∙
Cl∙ + Cl∙Cl∙ + H2 → H∙ + HCl
H∙ + Cl∙ → HCl

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 128 في هذه المجموعة.
فتح الحزمة
k this deck
43
Does one expect ΔS° in a propagation step of the free-radical chlorination of methane to be greater than zero, less than zero, or approximately equal to zero? Briefly explain your choice.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 128 في هذه المجموعة.
فتح الحزمة
k this deck
44
If stronger bonds are formed and weaker bonds are broken, then the reaction is ________.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 128 في هذه المجموعة.
فتح الحزمة
k this deck
45
Consider the elementary step in the solvolysis of isopropyl chloride shown below and write the rate equation for this step.
(CH3)2CHCl → (CH3)2CH+ + Cl-
(CH3)2CHCl → (CH3)2CH+ + Cl-

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 128 في هذه المجموعة.
فتح الحزمة
k this deck
46
The bond dissociation energy is the amount of energy required to break a bond ________.
A) homolytically
B) heterolytically
C) so as to produce the more stable pair of ions
D) via hydrogenation
E) none of the above
A) homolytically
B) heterolytically
C) so as to produce the more stable pair of ions
D) via hydrogenation
E) none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 128 في هذه المجموعة.
فتح الحزمة
k this deck
47
Will the sign of ΔS° in the combustion of propane be positive, negative or zero?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 128 في هذه المجموعة.
فتح الحزمة
k this deck
48
Given the bond dissociation energies below (in kcal/mol), calculate the overall ΔH° for the following reaction:
(CH3)3CH + Br2 → (CH3)3CBr + HBr
(CH3)3C-H 91
(CH3)3C-Br 65
Br-Br 46
H-Br 88
CH3-Br 70
(CH3)3CH + Br2 → (CH3)3CBr + HBr
(CH3)3C-H 91
(CH3)3C-Br 65
Br-Br 46
H-Br 88
CH3-Br 70

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 128 في هذه المجموعة.
فتح الحزمة
k this deck
49
Which compound has the smaller bond dissociation energy for its carbon-chlorine bond, CH3Cl or (CH3)3CCl? Explain your reasoning.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 128 في هذه المجموعة.
فتح الحزمة
k this deck
50
Draw the transition state for the hydrogen abstraction reaction shown below. (Hint: this is an endothermic reaction.)
(CH3)3C-H + ∙Br → (CH3)3C∙ + H-Br
(CH3)3C-H + ∙Br → (CH3)3C∙ + H-Br

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 128 في هذه المجموعة.
فتح الحزمة
k this deck
51
Which of the following is true for the termination step of a free radical chlorination reaction?
A) ΔH° > 0 and ΔS° > 0
B) ΔH° > 0 and ΔS° < 0
C) ΔH° < 0 and ΔS° > 0
D) ΔH° < 0 and ΔS° < 0
E) ΔH° = 0 and ΔS° = 0
A) ΔH° > 0 and ΔS° > 0
B) ΔH° > 0 and ΔS° < 0
C) ΔH° < 0 and ΔS° > 0
D) ΔH° < 0 and ΔS° < 0
E) ΔH° = 0 and ΔS° = 0

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 128 في هذه المجموعة.
فتح الحزمة
k this deck
52
Predict the signs of ΔH° and ΔS° in the reaction of cyclohexene with H2 to form cyclohexane, shown below. 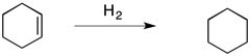


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 128 في هذه المجموعة.
فتح الحزمة
k this deck
53
Given the chlorination of acetone shown below, choose the correct rate law.
CH3COCH3 + Cl2 → CH3COCH2Cl + HCl
A) rate = [CH3COCH3]
B) rate = [Cl2]
C) rate = [CH3COCH3][Cl2]
D) rate = [CH3COCH3][Cl2]1/2
E) cannot be determined from stoichiometry; must be determined experimentally
CH3COCH3 + Cl2 → CH3COCH2Cl + HCl
A) rate = [CH3COCH3]
B) rate = [Cl2]
C) rate = [CH3COCH3][Cl2]
D) rate = [CH3COCH3][Cl2]1/2
E) cannot be determined from stoichiometry; must be determined experimentally

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 128 في هذه المجموعة.
فتح الحزمة
k this deck
54
Given the bond dissociation energies below (in kcal/mol), estimate the ΔH° for the propagation step 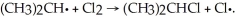 CH3CH2CH2-H 98
CH3CH2CH2-H 98
(CH3)2CH-H 95
Cl-Cl 58
H-Cl 103
CH3CH2CH2-Cl 81
(CH3)2CH-Cl 80
A) -22 kcal/mol
B) +22 kcal/mol
C) -40 kcal/mol
D) +45 kcal/mol
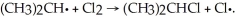 CH3CH2CH2-H 98
CH3CH2CH2-H 98(CH3)2CH-H 95
Cl-Cl 58
H-Cl 103
CH3CH2CH2-Cl 81
(CH3)2CH-Cl 80
A) -22 kcal/mol
B) +22 kcal/mol
C) -40 kcal/mol
D) +45 kcal/mol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 128 في هذه المجموعة.
فتح الحزمة
k this deck
55
What reactive species is produced in the initiation step of the free radical chlorination of 2,2-dimethylpropane?
A) a chlorine atom
B) a chlorine radical anion
C) a carbon radical
D) a carbocation
A) a chlorine atom
B) a chlorine radical anion
C) a carbon radical
D) a carbocation

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 128 في هذه المجموعة.
فتح الحزمة
k this deck
56
Energy is ________ when bonds are formed and is ________ when bonds are broken; therefore, bond dissociation energies are always ________.
A) released; consumed; exothermic
B) released; consumed; endothermic
C) consumed; released; exothermic
D) consumed; released; endothermic
E) consumed; released; isothermic
A) released; consumed; exothermic
B) released; consumed; endothermic
C) consumed; released; exothermic
D) consumed; released; endothermic
E) consumed; released; isothermic

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 128 في هذه المجموعة.
فتح الحزمة
k this deck
57
Consider the bond dissociation energies listed below in kcal/mol.
CH3-Br 70
CH3CH2-Br 68
(CH3)2CH-Br 68
(CH3)3C-Br 65
These data show that the carbon-bromine bond is weakest when bromine is bound to a ________.
A) methyl carbon
B) primary carbon
C) secondary carbon
D) tertiary carbon
E) quaternary carbon
CH3-Br 70
CH3CH2-Br 68
(CH3)2CH-Br 68
(CH3)3C-Br 65
These data show that the carbon-bromine bond is weakest when bromine is bound to a ________.
A) methyl carbon
B) primary carbon
C) secondary carbon
D) tertiary carbon
E) quaternary carbon

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 128 في هذه المجموعة.
فتح الحزمة
k this deck
58
Of the two C-H bonds shown, which has the smaller bond dissociation energy? Explain your choice.
(CH3)2CH-H vs. CH3CH2-H
(CH3)2CH-H vs. CH3CH2-H

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 128 في هذه المجموعة.
فتح الحزمة
k this deck
59
The hydrogenation of acetylene to produce ethane is shown below. Is ΔS° for this reaction positive, negative, or impossible to predict? Explain your reasoning.
C2H2 (g) + 2H2 (g) → C2H6 (g)
C2H2 (g) + 2H2 (g) → C2H6 (g)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 128 في هذه المجموعة.
فتح الحزمة
k this deck
60
Do you expect the initiation step in the free radical chlorination of 2,2-dimethylpropane to have a positive or negative ΔS? Explain briefly.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 128 في هذه المجموعة.
فتح الحزمة
k this deck
61
Given that the theoretical reaction below was found to be second order and bimolecular, provide a rate equation for the reaction.
A-B + C-D → A-C + B-D
A-B + C-D → A-C + B-D

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 128 في هذه المجموعة.
فتح الحزمة
k this deck
62
Consider the reaction energy diagram shown below. Label the axes.
a. Which points on the curve indicate transition states? Label them with A, B, etc.
b. Is the overall reaction exothermic or endothermic?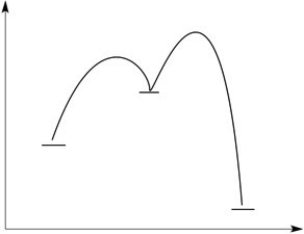
a. Which points on the curve indicate transition states? Label them with A, B, etc.
b. Is the overall reaction exothermic or endothermic?


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 128 في هذه المجموعة.
فتح الحزمة
k this deck
63
Explain the significance of the frequency factor A in the Arrhenius equation.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 128 في هذه المجموعة.
فتح الحزمة
k this deck
64
Consider the three-step mechanism for the reaction of A through intermediates B and C to produce D shown below.
A → B Ea = 15 kcal/mol, ΔH° = 13 kcal/mol
B → C Ea = 10 kcal/mol, ΔH° = -6 kcal/mol
C → D Ea = 2 kcal/mol, ΔH° = -20 kcal/mol
What's the enthalpy difference between reactant A and intermediate C?
A → B Ea = 15 kcal/mol, ΔH° = 13 kcal/mol
B → C Ea = 10 kcal/mol, ΔH° = -6 kcal/mol
C → D Ea = 2 kcal/mol, ΔH° = -20 kcal/mol
What's the enthalpy difference between reactant A and intermediate C?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 128 في هذه المجموعة.
فتح الحزمة
k this deck
65
Consider the reaction (CH3)3CBr + CH3CH2OH → 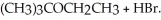 Experimentally one finds that if the concentration of (CH3)3CBr is tripled, the rate of the reaction triples. One also finds that if the concentration of CH3CH2OH is doubled, the rate of the reaction is unchanged. Which of the following correctly describes the kinetics of this reaction?
Experimentally one finds that if the concentration of (CH3)3CBr is tripled, the rate of the reaction triples. One also finds that if the concentration of CH3CH2OH is doubled, the rate of the reaction is unchanged. Which of the following correctly describes the kinetics of this reaction?
A) The reaction is third order in (CH3)3CBr.
B) The reaction is first order in CH3CH2OH.
C) The reaction is second order overall.
D) The reaction is first order overall.
E) none of the above
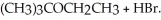 Experimentally one finds that if the concentration of (CH3)3CBr is tripled, the rate of the reaction triples. One also finds that if the concentration of CH3CH2OH is doubled, the rate of the reaction is unchanged. Which of the following correctly describes the kinetics of this reaction?
Experimentally one finds that if the concentration of (CH3)3CBr is tripled, the rate of the reaction triples. One also finds that if the concentration of CH3CH2OH is doubled, the rate of the reaction is unchanged. Which of the following correctly describes the kinetics of this reaction?A) The reaction is third order in (CH3)3CBr.
B) The reaction is first order in CH3CH2OH.
C) The reaction is second order overall.
D) The reaction is first order overall.
E) none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 128 في هذه المجموعة.
فتح الحزمة
k this deck
66
The rate of a reaction typically increases as the temperature increases because ________.
A) the A term in the Arrhenius equation increases
B) the fraction of molecules with kinetic energy greater than Ea increases
C) the activation energy decreases
D) the activation energy increases
E) the molecules make more collisions with the wall of the reaction vessel
A) the A term in the Arrhenius equation increases
B) the fraction of molecules with kinetic energy greater than Ea increases
C) the activation energy decreases
D) the activation energy increases
E) the molecules make more collisions with the wall of the reaction vessel

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 128 في هذه المجموعة.
فتح الحزمة
k this deck
67
The difference in energy between reactants and the transition state is known as ________.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 128 في هذه المجموعة.
فتح الحزمة
k this deck
68
Which of the following correctly expresses the standard Gibbs free energy change of a reaction in terms of the reaction temperature (T) and equilibrium constant (K)?
A) ΔG° = e-K/RT
B) ΔG° = eK/RT
C) ΔG° = RTlnK
D) ΔG° = -RTlnK
E) none of the above
A) ΔG° = e-K/RT
B) ΔG° = eK/RT
C) ΔG° = RTlnK
D) ΔG° = -RTlnK
E) none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 128 في هذه المجموعة.
فتح الحزمة
k this deck
69
The Arrhenius equation mathematically models which of the following statements?
A) The rate of a chemical reaction increases exponentially with increasing concentration of reactants.
B) The rate of a chemical reaction is directly related to the Ea and that increasing the temperature will alter the Ea for that reaction.
C) Increasing the temperature of a chemical reaction increases the number of particles in the reaction that have the minimum energy required to meet the Ea.
D) The rate of a chemical reaction is exponentially related to the Ea and relatively small differences in the Ea can dramatically affect the reaction rates of similar reactions at the same temperature.
E) both C and D
A) The rate of a chemical reaction increases exponentially with increasing concentration of reactants.
B) The rate of a chemical reaction is directly related to the Ea and that increasing the temperature will alter the Ea for that reaction.
C) Increasing the temperature of a chemical reaction increases the number of particles in the reaction that have the minimum energy required to meet the Ea.
D) The rate of a chemical reaction is exponentially related to the Ea and relatively small differences in the Ea can dramatically affect the reaction rates of similar reactions at the same temperature.
E) both C and D

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 128 في هذه المجموعة.
فتح الحزمة
k this deck
70
Consider the one-step conversion of F to G. Given that the reaction is endothermic by 5 kcal/mol and that the energy difference between G and the transition state for the process is 15 kcal/mol, sketch a reaction-energy diagram for this reaction. Make sure to show how the given energy differences are consistent with your sketch.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 128 في هذه المجموعة.
فتح الحزمة
k this deck
71
Rank the following carbocations in order of stability. (The most stable is first.) 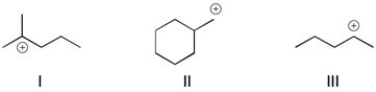
A) I > III > II
B) II > I > III
C) III > I > II
D) I > II > III

A) I > III > II
B) II > I > III
C) III > I > II
D) I > II > III

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 128 في هذه المجموعة.
فتح الحزمة
k this deck
72
________ is the minimum kinetic energy reacting molecules must possess to overcome the repulsions between their electron clouds when they collide.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 128 في هذه المجموعة.
فتح الحزمة
k this deck
73
In the hydrocarbon shown below, how many tertiary hydrogens are present? 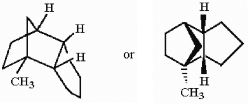
A) 0
B) 1
C) 2
D) 3
E) 4

A) 0
B) 1
C) 2
D) 3
E) 4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 128 في هذه المجموعة.
فتح الحزمة
k this deck
74
Consider the conversion of X to Z through the sole intermediate Y. Given the reaction-energy diagram shown below, which step is the rate-limiting step? Explain your reasoning. 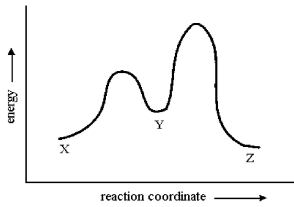


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 128 في هذه المجموعة.
فتح الحزمة
k this deck
75
Given an activation energy of 15 kcal/mol, use the Arrhenius equation to estimate how much faster the reaction will occur if the temperature is increased from 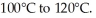
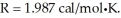
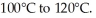
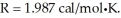

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 128 في هذه المجموعة.
فتح الحزمة
k this deck
76
What term describes the highest-energy structure in a molecular collision which leads to reaction?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 128 في هذه المجموعة.
فتح الحزمة
k this deck
77
Consider the reaction: CH3CH2∙ + Br2 → CH3CH2Br + Br∙ .
Given that this reaction has an activation energy of +6 kcal/mol and a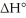
of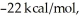
sketch a reaction-energy diagram for this reaction. Label the axes and show Ea and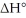
on your drawing.
Given that this reaction has an activation energy of +6 kcal/mol and a
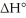
of
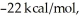
sketch a reaction-energy diagram for this reaction. Label the axes and show Ea and
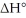
on your drawing.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 128 في هذه المجموعة.
فتح الحزمة
k this deck
78
Consider the three-step mechanism for the reaction of A through intermediates B and C to produce D shown below.
A → B Ea = 15 kcal/mol, ΔH° = 13 kcal/mol
B → C Ea = 10 kcal/mol, ΔH° = -6 kcal/mol
C → D Ea = 2 kcal/mol, ΔH° = -20 kcal/mol
Which of the three steps is rate-limiting?
A) The reaction of A to B.
B) The reaction of B to C.
C) The reaction of C to D.
D) All three steps occur at the same rate; there is no rate-limiting step.
E) The most exothermic step is rate-limiting.
A → B Ea = 15 kcal/mol, ΔH° = 13 kcal/mol
B → C Ea = 10 kcal/mol, ΔH° = -6 kcal/mol
C → D Ea = 2 kcal/mol, ΔH° = -20 kcal/mol
Which of the three steps is rate-limiting?
A) The reaction of A to B.
B) The reaction of B to C.
C) The reaction of C to D.
D) All three steps occur at the same rate; there is no rate-limiting step.
E) The most exothermic step is rate-limiting.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 128 في هذه المجموعة.
فتح الحزمة
k this deck
79
The following reaction occurs readily: 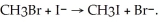
Experimentally one finds that if the concentration of I- is doubled, the rate doubles. Also if the concentration of CH3Br is halved, the rate is halved. What is the rate equation for this reaction?
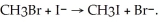
Experimentally one finds that if the concentration of I- is doubled, the rate doubles. Also if the concentration of CH3Br is halved, the rate is halved. What is the rate equation for this reaction?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 128 في هذه المجموعة.
فتح الحزمة
k this deck
80
Provide the structure of the transition state in the first propagation step of the free radical chlorination of ethane.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 128 في هذه المجموعة.
فتح الحزمة
k this deck








