Deck 15: Benzene and Aromaticity: Electrophilic Aromatic Substitution
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/29
العب
ملء الشاشة (f)
Deck 15: Benzene and Aromaticity: Electrophilic Aromatic Substitution
1
Which of the following carbocations would you expect to be most stable?
A)
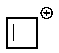
B)
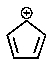
C)
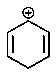
D)
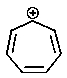
E)
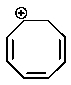
A)

B)

C)

D)

E)


2
How would you name the following? 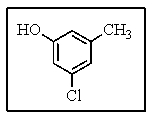
A) 3-chloro-5-methylphenol
B) 3-hydroxy-5-chlorotoluene
C) 1-chloro-3-hydroxy-5-methylbenzene
D) Meta-methy-meta-hydroxytoluene
E) 3-hydroxy-5-methylchlorobenzene

A) 3-chloro-5-methylphenol
B) 3-hydroxy-5-chlorotoluene
C) 1-chloro-3-hydroxy-5-methylbenzene
D) Meta-methy-meta-hydroxytoluene
E) 3-hydroxy-5-methylchlorobenzene
3-chloro-5-methylphenol
3
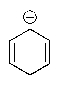
Which of the following would not be aromatic (i.e.,which would not be unusually stable)?
A)
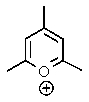
B)
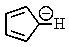
C)
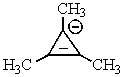
D)
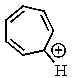
E)
A)

B)

C)

D)

E)


4
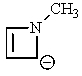
Which of the following molecules would you predict to be aromatic?
A)
B)
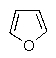
C)

D)
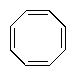
E)
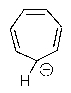
A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 29 في هذه المجموعة.
فتح الحزمة
k this deck
5
What is the common name for the following compound? 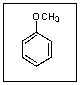
A) Toluene
B) Anisole
C) Aniline
D) Phenol
E) Cresol

A) Toluene
B) Anisole
C) Aniline
D) Phenol
E) Cresol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 29 في هذه المجموعة.
فتح الحزمة
k this deck
6
The best experimental proof of aromaticity is
A) a pleasant odor.
B) infrared CH absorption frequency.
C) NMR chemical shifts.
D) presence of resonance structures.
E) presence of conjugation.
A) a pleasant odor.
B) infrared CH absorption frequency.
C) NMR chemical shifts.
D) presence of resonance structures.
E) presence of conjugation.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 29 في هذه المجموعة.
فتح الحزمة
k this deck
7
Which of the following is not a resonance structure involved in electrophilic aromatic bromination?
A)
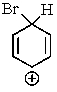
B)
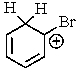
C)
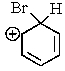
D)
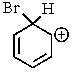
E) All of these are valid resonance structures.
A)

B)

C)

D)

E) All of these are valid resonance structures.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 29 في هذه المجموعة.
فتح الحزمة
k this deck
8
What would be the expected product of the following reactions? 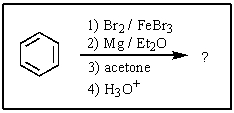
A)
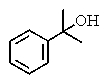
B)
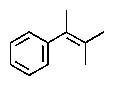
C)
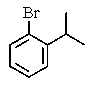
D)
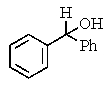
E)
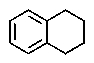
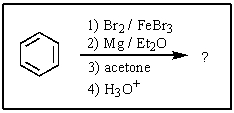
A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 29 في هذه المجموعة.
فتح الحزمة
k this deck
9
Which of the following would you expect to be aromatic?
A)
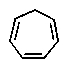
B)
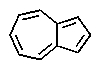
C)
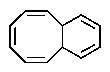
D)
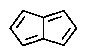
E)
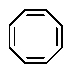
A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 29 في هذه المجموعة.
فتح الحزمة
k this deck
10
Which of the following reactions of aromatics is reversible?
A) Nitration
B) Bromination
C) F-C alkylation
D) Sulfonation
E) F-C acylation
A) Nitration
B) Bromination
C) F-C alkylation
D) Sulfonation
E) F-C acylation

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 29 في هذه المجموعة.
فتح الحزمة
k this deck
11
Which of the following reactions has an aromatic transition state with (4n + 2)electrons involved?
A)
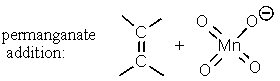
B)
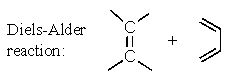
C)
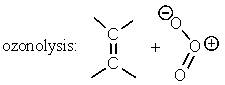
D) Two of these.
E) All of these.
A)

B)

C)

D) Two of these.
E) All of these.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 29 في هذه المجموعة.
فتح الحزمة
k this deck
12
What reagents are typically required to accomplish bromination of an aromatic ring?
A) Br2 + HBr
B) Br2 + heat
C) Br2 + light
D) Br2 + FeBr3
E) Br2 + H2SO4
A) Br2 + HBr
B) Br2 + heat
C) Br2 + light
D) Br2 + FeBr3
E) Br2 + H2SO4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 29 في هذه المجموعة.
فتح الحزمة
k this deck
13
How many mononitrated (on the aromatic ring)derivatives of meta-xylene (= 1,3-dimethylbenzene)are possible? (Don't worry about how they might be made or which would be the major product.)
A) 1
B) 2
C) 3
D) 4
E) 5
A) 1
B) 2
C) 3
D) 4
E) 5

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 29 في هذه المجموعة.
فتح الحزمة
k this deck
14
Which of the following would you expect to react fastest in an SN1 reaction (consider the mechanism)?
A)
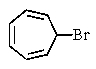
B)
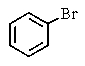
C)
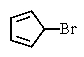
D) All would react rapidly.
E) None would react.
A)

B)

C)

D) All would react rapidly.
E) None would react.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 29 في هذه المجموعة.
فتح الحزمة
k this deck
15
What is/are the major product(s)in the following reaction? 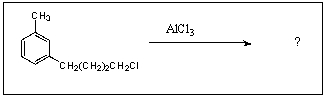
A)
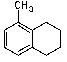
B)
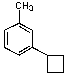
C)
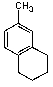
D) Both A and C
E) No Reaction

A)

B)

C)

D) Both A and C
E) No Reaction

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 29 في هذه المجموعة.
فتح الحزمة
k this deck
16
Which would be the best systematic name of vanillan,the primary flavoring ingredient in vanilla? 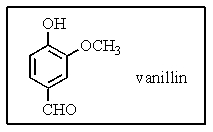
A) 4-formyl-2-methoxyphenol
B) 3-formyl-6-hydroxyanisole
C) 4-hydroxy-3-methoxybenzaldehyde
D) 5-formyl-2-hydroxyanisole
E) 4-formyl-5-methoxyphenol

A) 4-formyl-2-methoxyphenol
B) 3-formyl-6-hydroxyanisole
C) 4-hydroxy-3-methoxybenzaldehyde
D) 5-formyl-2-hydroxyanisole
E) 4-formyl-5-methoxyphenol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 29 في هذه المجموعة.
فتح الحزمة
k this deck
17
The reagents listed below all react readily with alkenes.Which one can react (slowly)irreversibly with an aromatic ring?
A) HBr + peroxides
B) CH3CO3H
C) HBr
D) H2/catalyst
E) H2O,H+
A) HBr + peroxides
B) CH3CO3H
C) HBr
D) H2/catalyst
E) H2O,H+

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 29 في هذه المجموعة.
فتح الحزمة
k this deck
18
One of the double bonds in phenanthrene,shown below,is much more reactive than the others.Which one is it? 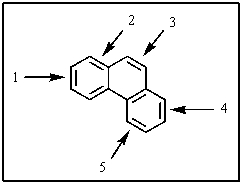
A) 1
B) 2
C) 3
D) 4
E) 5

A) 1
B) 2
C) 3
D) 4
E) 5

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 29 في هذه المجموعة.
فتح الحزمة
k this deck
19
Which of the following best describes the electronic nature of cyclooctatetraene? 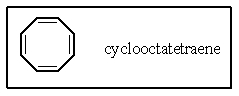
A) This compound is predicted to be aromatic.
B) This compound is anti-aromatic and very unstable.
C) This compound is non-planar and non-aromatic.
D) Not all of the carbon atoms possess the -orbital required for conjugation.
E) None of these are true.

A) This compound is predicted to be aromatic.
B) This compound is anti-aromatic and very unstable.
C) This compound is non-planar and non-aromatic.
D) Not all of the carbon atoms possess the -orbital required for conjugation.
E) None of these are true.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 29 في هذه المجموعة.
فتح الحزمة
k this deck
20
What would be the product of the following reaction? 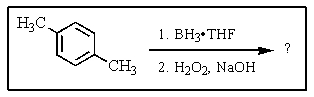
A)
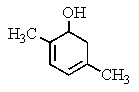
B)
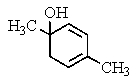
C)
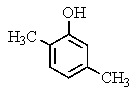
D)
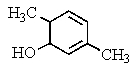
E) No reaction occurs.

A)

B)

C)

D)

E) No reaction occurs.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 29 في هذه المجموعة.
فتح الحزمة
k this deck
21
What is the major product of the following reaction? 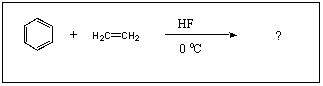
A)
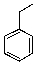
B)
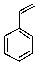
C)
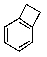
D)
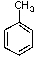
E) no reaction

A)

B)

C)

D)

E) no reaction

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 29 في هذه المجموعة.
فتح الحزمة
k this deck
22
The infrared spectrum of a dimethylbenzene has a band at 738 cm-1.What is the most likely structure of the compound?
A) 1,2-dimethylbenzene
B) 1,3-dimethylbenzene
C) 1,4-dimethylbenzene
D) 1,5-dimethylbenzene
E) Not enough information to determine
A) 1,2-dimethylbenzene
B) 1,3-dimethylbenzene
C) 1,4-dimethylbenzene
D) 1,5-dimethylbenzene
E) Not enough information to determine

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 29 في هذه المجموعة.
فتح الحزمة
k this deck
23
Which of the following statements are not true of benzene?
A) The carbon-carbon bond lengths alternated between single bonds and double bonds.
B) All carbons are sp2 hybridized.
C) The delocalized electrons form a circular pi-cloud above and below the ring.
D) It is especially stable according to heats of hydrogenation data.
E) None of the above.All of these statements are true.
A) The carbon-carbon bond lengths alternated between single bonds and double bonds.
B) All carbons are sp2 hybridized.
C) The delocalized electrons form a circular pi-cloud above and below the ring.
D) It is especially stable according to heats of hydrogenation data.
E) None of the above.All of these statements are true.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 29 في هذه المجموعة.
فتح الحزمة
k this deck
24
What is/are the product(s)in the following Friedel-Crafts Reaction? 
A)
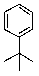
B)
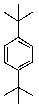
C)

D) Both A and B.
E) All of the above.

A)

B)

C)

D) Both A and B.
E) All of the above.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 29 في هذه المجموعة.
فتح الحزمة
k this deck
25
Which of the following is not an allotrope of carbon?
A) Graphite
B) Diamond
C) Coal
D) C60
E) All are allotropes of carbon.
A) Graphite
B) Diamond
C) Coal
D) C60
E) All are allotropes of carbon.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 29 في هذه المجموعة.
فتح الحزمة
k this deck
26
If the heat of hydrogenation of the hypothetical 1,3,5-cyclohexatriene is -78.9 kcal/mol,but the heat of hydrogenation of benzene is -49.3 kcal/mol,what is the stabilization energy attributed to the resonance in benzene?
A) -49.3 kcal/mol + (-78.9 kcal/mol)= -128.2 kcal/mol
B) -49.3 kcal/mol - (-78.9 kcal/mol)= 29.6 kcal/mol
C) -49.3 kcal/mol
D) -78.9 kcal/mol
E) None of the above.
A) -49.3 kcal/mol + (-78.9 kcal/mol)= -128.2 kcal/mol
B) -49.3 kcal/mol - (-78.9 kcal/mol)= 29.6 kcal/mol
C) -49.3 kcal/mol
D) -78.9 kcal/mol
E) None of the above.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 29 في هذه المجموعة.
فتح الحزمة
k this deck
27
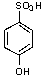
What is/are the product(s)from the following reaction? 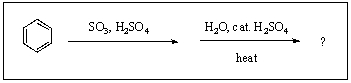
A)
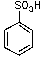
B)
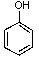
C)
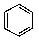
D)
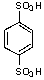
E)

A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 29 في هذه المجموعة.
فتح الحزمة
k this deck
28
Which of the following mechanisms best describes electrophilic aromatic substitution of benzene with bromine?
A)
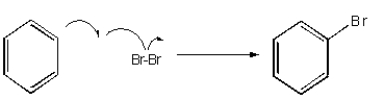
B)
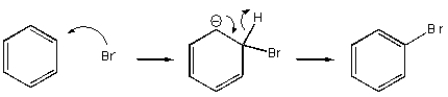
C)
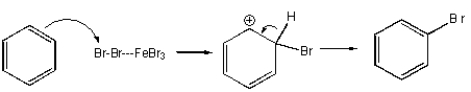
D)
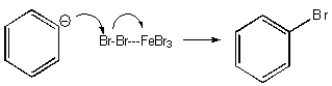
E) None of the above.
A)

B)

C)

D)

E) None of the above.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 29 في هذه المجموعة.
فتح الحزمة
k this deck
29
Which of the following molecular orbitals corresponds to the 1 molecular orbital of benzene?
A)
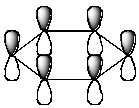
B)
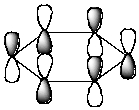
C)
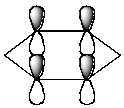
D)
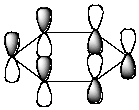
E) None of the above.
A)

B)

C)

D)

E) None of the above.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 29 في هذه المجموعة.
فتح الحزمة
k this deck








