Deck 1: Structure and Bonding in Organic Molecules
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/50
العب
ملء الشاشة (f)
Deck 1: Structure and Bonding in Organic Molecules
1
In the following molecule,how many carbon atoms are in the sp3 hybridization state? 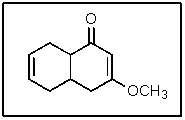
A) 2
B) 4
C) 5
D) 6
E) 11

A) 2
B) 4
C) 5
D) 6
E) 11
6
2
Which of the following pairs are not resonance structures of each other?
A)
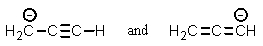
B)
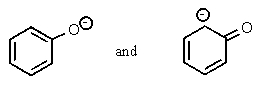
C)
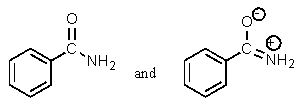
D)
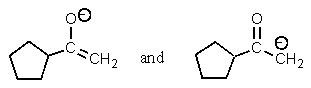
E) All are pairs of resonance structures.
A)
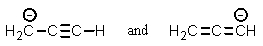
B)
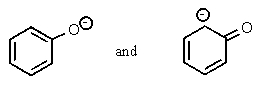
C)

D)
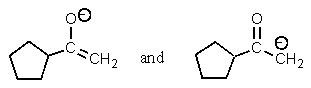
E) All are pairs of resonance structures.
All are pairs of resonance structures.
3
The lone-pair of electrons on nitrogen in the following molecule reside in what type of orbital? 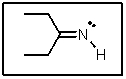
A) sp3
B) sp2
C) sp
D) 2p
E) 2s

A) sp3
B) sp2
C) sp
D) 2p
E) 2s
sp2
4
In the following molecule,how many carbon atoms are in the sp2 hybridization state? 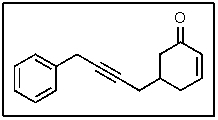
A) 1
B) 3
C) 7
D) 8
E) 9

A) 1
B) 3
C) 7
D) 8
E) 9

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 50 في هذه المجموعة.
فتح الحزمة
k this deck
5
The following molecule contains how many carbon atoms in the sp hybridization state? 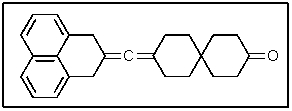
A) 1
B) 3
C) 8
D) 13
E) 16

A) 1
B) 3
C) 8
D) 13
E) 16

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 50 في هذه المجموعة.
فتح الحزمة
k this deck
6
What is the molecular formula of limonene,the major volatile compound in orange peel oil? 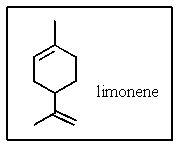
A) C10H18
B) C10H20
C) C10H16
D) C11H14
E) C11H18

A) C10H18
B) C10H20
C) C10H16
D) C11H14
E) C11H18

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 50 في هذه المجموعة.
فتح الحزمة
k this deck
7
A positive charge on oxygen generally occurs when:
A) oxygen has too many electrons.
B) oxygen has too few electrons.
C) oxygen is sharing one of its non-bonding electron pairs.
D) oxygen has too many non-bonding electron pairs.
E) oxygen is borrowing electrons from another atom.
A) oxygen has too many electrons.
B) oxygen has too few electrons.
C) oxygen is sharing one of its non-bonding electron pairs.
D) oxygen has too many non-bonding electron pairs.
E) oxygen is borrowing electrons from another atom.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 50 في هذه المجموعة.
فتح الحزمة
k this deck
8
What would be the ideal value for the indicated bond angle? 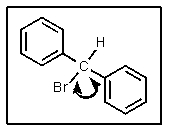
A) 120
B) 90
C) 104
D) 180
E) 109

A) 120
B) 90
C) 104
D) 180
E) 109

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 50 في هذه المجموعة.
فتح الحزمة
k this deck
9
Which of the following is not a resonance structure of the others?
A)
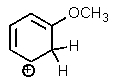
B)
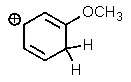
C)
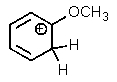
D)
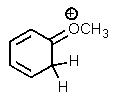
E)
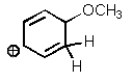
A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 50 في هذه المجموعة.
فتح الحزمة
k this deck
10
Which one of the following structures is not chemically identical to the others?
A)
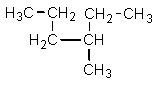
B)
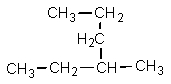
C)
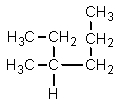
D)
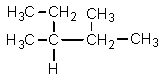
E)
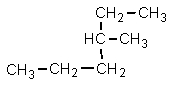
A)
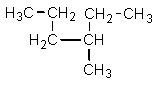
B)
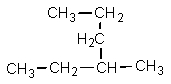
C)

D)
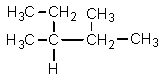
E)
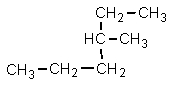

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 50 في هذه المجموعة.
فتح الحزمة
k this deck
11
Which one of the following structures must be incorrect?
A)
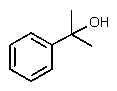
B)

C)
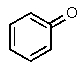
D)
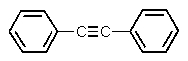
E)
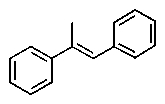
A)

B)

C)

D)
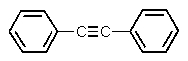
E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 50 في هذه المجموعة.
فتح الحزمة
k this deck
12
Which structure matches the following condensed structure? 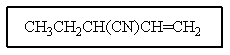
A)
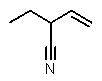
B)
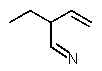
C)
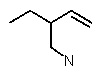
D)
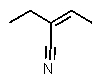
E)
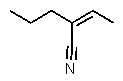

A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 50 في هذه المجموعة.
فتح الحزمة
k this deck
13
The boxed item most likely represents what? 
A) s orbital
B) sp3 orbital
C) p orbital
D) could be any of A-C
E) None of the above.

A) s orbital
B) sp3 orbital
C) p orbital
D) could be any of A-C
E) None of the above.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 50 في هذه المجموعة.
فتح الحزمة
k this deck
14
In the following molecule,how many carbon atoms are in the sp2 hybridization state? 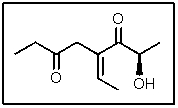
A) 0
B) 1
C) 2
D) 4
E) 6

A) 0
B) 1
C) 2
D) 4
E) 6

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 50 في هذه المجموعة.
فتح الحزمة
k this deck
15
How many atoms in ethene are required by sp2 bonding to lie in the same plane? 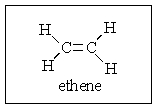
A) 2
B) 3
C) 4
D) 5
E) 6

A) 2
B) 3
C) 4
D) 5
E) 6

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 50 في هذه المجموعة.
فتح الحزمة
k this deck
16
Which one of the resonance structures below would be the most important (i.e.,most stable)?
A)
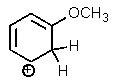
B)
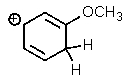
C)
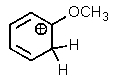
D)
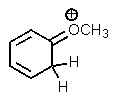
E)
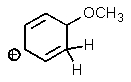
A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 50 في هذه المجموعة.
فتح الحزمة
k this deck
17
The nitrogen of trimethylamine [(CH3)3N] contains how many lone pairs of electrons?
A) none
B) one
C) two
D) three
E) there is no nitrogen in this molecule
A) none
B) one
C) two
D) three
E) there is no nitrogen in this molecule

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 50 في هذه المجموعة.
فتح الحزمة
k this deck
18
Of those indicated,which would be the shortest carbon-carbon bond in -selinene? 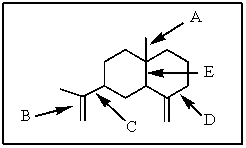
A) A
B) B
C) C
D) D
E) E

A) A
B) B
C) C
D) D
E) E

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 50 في هذه المجموعة.
فتح الحزمة
k this deck
19
In the following molecule,how many carbon atoms are in the sp hybridization state? 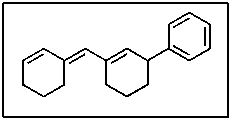
A) 2
B) 4
C) 6
D) 12
E) None of the above.

A) 2
B) 4
C) 6
D) 12
E) None of the above.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 50 في هذه المجموعة.
فتح الحزمة
k this deck
20
How many hydrogen atoms are part of the following steroid? 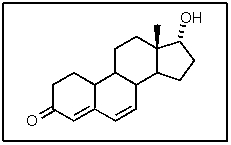
A) 18
B) 20
C) 21
D) 22
E) 24

A) 18
B) 20
C) 21
D) 22
E) 24

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 50 في هذه المجموعة.
فتح الحزمة
k this deck
21
What is the molecular formula of carvone,the major volatile compound in caraway oil? 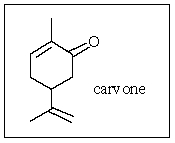
A) C10H18O
B) C10H17O
C) C10H16O
D) C10H14O
E) C10H15O

A) C10H18O
B) C10H17O
C) C10H16O
D) C10H14O
E) C10H15O

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 50 في هذه المجموعة.
فتح الحزمة
k this deck
22
Which one of the following structures must be incorrect?
A)
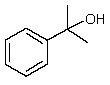
B)
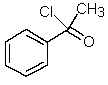
C)
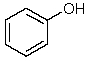
D)
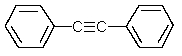
E)
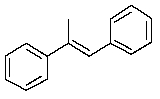
A)

B)

C)

D)
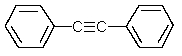
E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 50 في هذه المجموعة.
فتح الحزمة
k this deck
23
Which of the carbon-carbon bonds indicated would you expect to be the longest in stilbene? 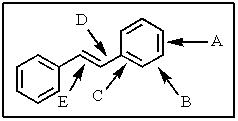
A) A
B) B
C) C
D) D
E) E

A) A
B) B
C) C
D) D
E) E

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 50 في هذه المجموعة.
فتح الحزمة
k this deck
24
The molecular formula for piperitone is 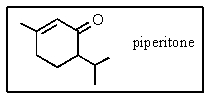
A) C9H16O
B) C10H18O
C) C9H18O
D) C10H14O
E) C10H16O

A) C9H16O
B) C10H18O
C) C9H18O
D) C10H14O
E) C10H16O

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 50 في هذه المجموعة.
فتح الحزمة
k this deck
25
In the following molecule,how many carbon atoms are in the sp3 hybridization state? 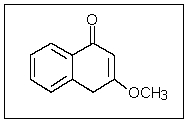
A) 2
B) 4
C) 5
D) 6
E) 9

A) 2
B) 4
C) 5
D) 6
E) 9

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 50 في هذه المجموعة.
فتح الحزمة
k this deck
26
Which structure is different from the others?
A)
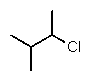
B) CH3CHClCH(CH3)2
C)
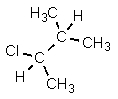
D)
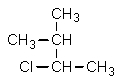
E) All are identical.
A)

B) CH3CHClCH(CH3)2
C)

D)

E) All are identical.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 50 في هذه المجموعة.
فتح الحزمة
k this deck
27
Of those indicated,which would be the shortest carbon-carbon bond in -cadinene? 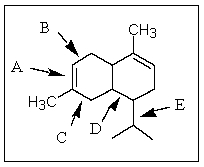
A) A
B) B
C) C
D) D
E) E

A) A
B) B
C) C
D) D
E) E

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 50 في هذه المجموعة.
فتح الحزمة
k this deck
28
Which of the following pairs are not resonance structures of each other?
A)
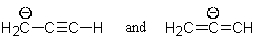
B)
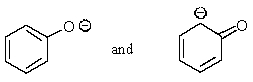
C)
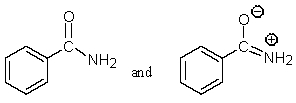
D)
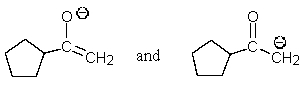
E) All are pairs of resonance structures.
A)
B)
C)

D)
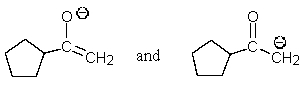
E) All are pairs of resonance structures.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 50 في هذه المجموعة.
فتح الحزمة
k this deck
29
Which of the following molecules are most likely to be held together by a purely covalent bond?
A) NaCl
B) H2
C) HF
D) BH3
E) KI
A) NaCl
B) H2
C) HF
D) BH3
E) KI

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 50 في هذه المجموعة.
فتح الحزمة
k this deck
30
What would be the ideal value for the indicated bond angle? 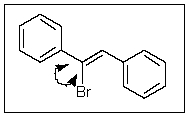
A) 120
B) 90
C) 104
D) 180
E) 109

A) 120
B) 90
C) 104
D) 180
E) 109

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 50 في هذه المجموعة.
فتح الحزمة
k this deck
31
Which structure matches the following condensed structure? 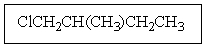
A)
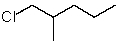
B)
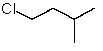
C)
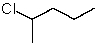
D)
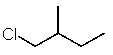
E)
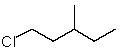
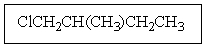
A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 50 في هذه المجموعة.
فتح الحزمة
k this deck
32
The following molecule has what molecular formula? 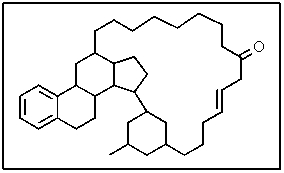
A) C39H58O
B) C40H58O
C) C39H60O
D) C44H44O
E) None of the above.

A) C39H58O
B) C40H58O
C) C39H60O
D) C44H44O
E) None of the above.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 50 في هذه المجموعة.
فتح الحزمة
k this deck
33
Which of the following statements are true of sp orbitals?
A) Orbitals of the sp type are 50% s and 50% p character.
B) They are hybrid orbitals.
C) They are linear.
D) They result when one s orbital and one p orbital are mixed.
E) All are correct.
A) Orbitals of the sp type are 50% s and 50% p character.
B) They are hybrid orbitals.
C) They are linear.
D) They result when one s orbital and one p orbital are mixed.
E) All are correct.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 50 في هذه المجموعة.
فتح الحزمة
k this deck
34
The carbon atom in CH2Cl2 has what hybridization?
A) sp
B) sp2
C) sp3
D) sp4
E) they are not hybridized
A) sp
B) sp2
C) sp3
D) sp4
E) they are not hybridized

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 50 في هذه المجموعة.
فتح الحزمة
k this deck
35
How many sp2 hybridized carbon atoms are in the potent anticancer compound hydroxymethylacylfulvene? 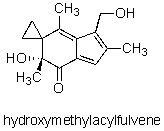
A) 2
B) 4
C) 6
D) 8
E) None of the above.

A) 2
B) 4
C) 6
D) 8
E) None of the above.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 50 في هذه المجموعة.
فتح الحزمة
k this deck
36
What is the molecular formula of camphor? 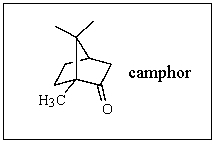
A) C10H15O
B) C10H16O
C) C10H17O
D) C11H18O
E) C11H16O

A) C10H15O
B) C10H16O
C) C10H17O
D) C11H18O
E) C11H16O

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 50 في هذه المجموعة.
فتح الحزمة
k this deck
37
A fairly common algal metabolite is the compound (-)-geosmin,which imparts a musty odor to water even at concentrations in the ppb range.What is the molecular formula of geosmin? 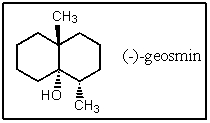
A) C11H20O
B) C12H22O
C) C11H21O
D) C12H20O
E) C12H21O

A) C11H20O
B) C12H22O
C) C11H21O
D) C12H20O
E) C12H21O

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 50 في هذه المجموعة.
فتح الحزمة
k this deck
38
Camptothecin is an important anticancer compound; how many carbons are in the sp hybridization state? 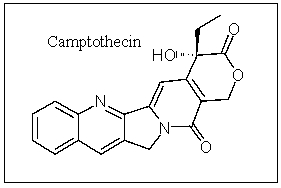
A) 0
B) 1
C) 2
D) 3
E) 4

A) 0
B) 1
C) 2
D) 3
E) 4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 50 في هذه المجموعة.
فتح الحزمة
k this deck
39
The process of adding electrons one by one to atomic orbitals beginning with the lowest energy is described by:
A) the Aufbau Principle.
B) Hund's Rule.
C) the de Broglie Relation.
D) the Pauli Exclusion Principle.
E) Coulomb's Law.
A) the Aufbau Principle.
B) Hund's Rule.
C) the de Broglie Relation.
D) the Pauli Exclusion Principle.
E) Coulomb's Law.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 50 في هذه المجموعة.
فتح الحزمة
k this deck
40
How many sp3 carbons are in the following molecule? 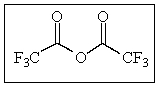
A) 0
B) 1
C) 2
D) 3
E) 4

A) 0
B) 1
C) 2
D) 3
E) 4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 50 في هذه المجموعة.
فتح الحزمة
k this deck
41
Which of the following cannot be a correct Lewis structure?
A)
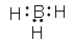
B)
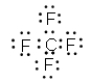
C)
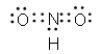
D)
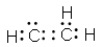
E) All are correct.
A)
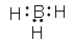
B)

C)
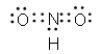
D)
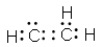
E) All are correct.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 50 في هذه المجموعة.
فتح الحزمة
k this deck
42
The following molecule belongs to a class of compounds known as allenes.Based on your knowledge of bonding,predict the hybridization of the carbon atom indicated by the arrow. 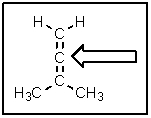
A) sp
B) sp2
C) sp3
D) p-p pi
E) a hypervalent carbon

A) sp
B) sp2
C) sp3
D) p-p pi
E) a hypervalent carbon

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 50 في هذه المجموعة.
فتح الحزمة
k this deck
43
A hydrocarbon with a double bond and a ring will have the general formula?
A) CnH2n+2
B) CnH2n
C) CnH2n-2
D) CnH2n-4
E) C2nH2n
A) CnH2n+2
B) CnH2n
C) CnH2n-2
D) CnH2n-4
E) C2nH2n

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 50 في هذه المجموعة.
فتح الحزمة
k this deck
44
Which of the following represent resonance contributing Lewis structures for CH2N2?
A)
B)
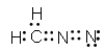
C)
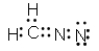
D)
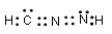
E) both A and B are correct
A)
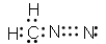
B)
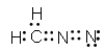
C)
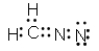
D)
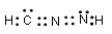
E) both A and B are correct

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 50 في هذه المجموعة.
فتح الحزمة
k this deck
45
Which of the following most correctly defines "structural isomers"?
A) molecules with different molecular formulas but the same connectivity
B) compounds that are not constitutional isomers
C) molecules with the same molecular formula but different connectivity
D) Anti and gauche conformers
E) both B and C
A) molecules with different molecular formulas but the same connectivity
B) compounds that are not constitutional isomers
C) molecules with the same molecular formula but different connectivity
D) Anti and gauche conformers
E) both B and C

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 50 في هذه المجموعة.
فتح الحزمة
k this deck
46
How many structural isomers exist for the formula C6H14?
A) 3
B) 4
C) 5
D) 6
E) 7
A) 3
B) 4
C) 5
D) 6
E) 7

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 50 في هذه المجموعة.
فتح الحزمة
k this deck
47
How many isomers of C5H12 are possible?
A) two
B) three
C) four
D) five
E) six
A) two
B) three
C) four
D) five
E) six

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 50 في هذه المجموعة.
فتح الحزمة
k this deck
48
How many different resonance structures can be drawn for the benzyl cation (shown below)which place the plus charge on a carbon atom in the ring? 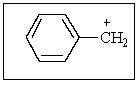
A) 1
B) 2
C) 3
D) 5
E) 6

A) 1
B) 2
C) 3
D) 5
E) 6

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 50 في هذه المجموعة.
فتح الحزمة
k this deck
49
How many isomers of C4H9Br are possible?
A) two
B) three
C) four
D) five
E) six
A) two
B) three
C) four
D) five
E) six

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 50 في هذه المجموعة.
فتح الحزمة
k this deck
50
What is the hybridization of the each of the labeled atoms for the potent neurotoxin (-)-gephyrotoxin? 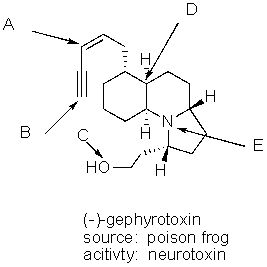
A) A = sp2,B = sp,C = sp2,D = sp3,E = sp3
B) A = sp2,B = sp,C = sp3,D = sp3,E = sp2
C) A = sp2,B = sp,C = sp2,D = sp3,E = sp2
D) A = sp2,B = sp,C = sp3,D = sp3,E = sp3
E) A = sp,B = sp,C = sp3,D = sp3,E = sp3

A) A = sp2,B = sp,C = sp2,D = sp3,E = sp3
B) A = sp2,B = sp,C = sp3,D = sp3,E = sp2
C) A = sp2,B = sp,C = sp2,D = sp3,E = sp2
D) A = sp2,B = sp,C = sp3,D = sp3,E = sp3
E) A = sp,B = sp,C = sp3,D = sp3,E = sp3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 50 في هذه المجموعة.
فتح الحزمة
k this deck








