Deck 10: Structure Determination: Mass Spectrometry, Infrared Spectroscopy, and Ultraviolet Spectroscopy
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/43
العب
ملء الشاشة (f)
Deck 10: Structure Determination: Mass Spectrometry, Infrared Spectroscopy, and Ultraviolet Spectroscopy
1
What is the horizontal axis of a mass spectrum?
A) mass
B) mass/energy
C) mass/charge
D) charge
A) mass
B) mass/energy
C) mass/charge
D) charge
mass/charge
2
The amount of energy in infrared light corresponds to:
A) the amount of energy needed to promote one electron from a bonding to an antibonding molecular orbital
B) the amount of energy needed to fragment a molecule
C) the amount of energy needed to strip a molecule of one electron to generate a cation radical
D) the amount of energy needed to increase certain molecular motions, such as bond vibrations, in organic molecules
A) the amount of energy needed to promote one electron from a bonding to an antibonding molecular orbital
B) the amount of energy needed to fragment a molecule
C) the amount of energy needed to strip a molecule of one electron to generate a cation radical
D) the amount of energy needed to increase certain molecular motions, such as bond vibrations, in organic molecules
the amount of energy needed to increase certain molecular motions, such as bond vibrations, in organic molecules
3
The amount of energy in electromagnetic radiation is related to the frequency and wavelength of the radiation. High energy radiation, like gamma rays, is of:
A) low frequency and short wavelength
B) low frequency and long wavelength
C) high frequency and short wavelength
D) high frequency and long wavelength
A) low frequency and short wavelength
B) low frequency and long wavelength
C) high frequency and short wavelength
D) high frequency and long wavelength
high frequency and short wavelength
4
Which of the following regions in the electromagnetic spectrum corresponds to the radiation with the highest energy?
A) radio waves
B) ultraviolet
C) infrared
D) visible
A) radio waves
B) ultraviolet
C) infrared
D) visible

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 43 في هذه المجموعة.
فتح الحزمة
k this deck
5
Which of the following compounds gives an infrared spectrum with a peak at strong 1730 cm−1?
A)
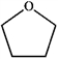
B)
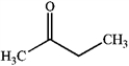
C)
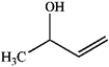
D)
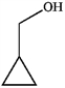
A)

B)

C)

D)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 43 في هذه المجموعة.
فتح الحزمة
k this deck
6
Which of the following bonds undergoes stretching at the highest frequency?
A) C=O
B) C−O
C) C=C
D) C−C
A) C=O
B) C−O
C) C=C
D) C−C

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 43 في هذه المجموعة.
فتح الحزمة
k this deck
7
Which of the following compounds gives an infrared spectrum with no significant peaks at ˜3300 cm−1 or 1600−1680 cm−1?
A)
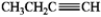
B)

C)
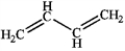
D)
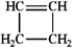
A)
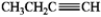
B)

C)

D)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 43 في هذه المجموعة.
فتح الحزمة
k this deck
8
Which of the following bonds undergoes stretching at the highest frequency?
A) O−H
B) C−H
C) C=O
D) C
C
A) O−H
B) C−H
C) C=O
D) C
C

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 43 في هذه المجموعة.
فتح الحزمة
k this deck
9
Which of the following statements best describes the base peak in a mass spectrum?
A) The peak from the most stable radical.
B) The peak from the species that has the isotope with the highest atomic number.
C) The peak of highest intensity.
D) The peak from the molecule minus an electron.
E) The M +1 peak
A) The peak from the most stable radical.
B) The peak from the species that has the isotope with the highest atomic number.
C) The peak of highest intensity.
D) The peak from the molecule minus an electron.
E) The M +1 peak

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 43 في هذه المجموعة.
فتح الحزمة
k this deck
10
Which of the following does not involve the interaction of molecules with electromagnetic energy?
A) mass spectrometry
B) infrared spectroscopy
C) ultraviolet spectroscopy
D) nuclear magnetic resonance spectroscopy
A) mass spectrometry
B) infrared spectroscopy
C) ultraviolet spectroscopy
D) nuclear magnetic resonance spectroscopy

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 43 في هذه المجموعة.
فتح الحزمة
k this deck
11
What are the units for electromagnetic radiation used in infrared spectroscopy?
A) cm
B) cm−1
C) J.mol−1
D) none, wavenumber is a dimensionless quantity
A) cm
B) cm−1
C) J.mol−1
D) none, wavenumber is a dimensionless quantity

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 43 في هذه المجموعة.
فتح الحزمة
k this deck
12
Which of the following compounds gives an infrared spectrum with peaks at 3300 cm−1 (sharp peak) and 2150 cm−1 (sharp peak)?
A)
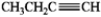
B)

C)
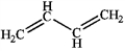
D)
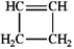
A)
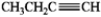
B)

C)

D)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 43 في هذه المجموعة.
فتح الحزمة
k this deck
13
Select the most reasonable formula for the compounds with the following mass spectral data.
Refer to instructions. M+ at m/z = 101
A) C5H6Br
B) C5H12N2
C) C6H15N
D) C9H12O
Refer to instructions. M+ at m/z = 101
A) C5H6Br
B) C5H12N2
C) C6H15N
D) C9H12O

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 43 في هذه المجموعة.
فتح الحزمة
k this deck
14
Which of the following compounds gives an infrared spectrum with a peak at ˜1750 cm−1, but no significant peaks at 3000−3500 cm−1 or 1050−1250 cm−1?
A)
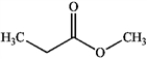
B)
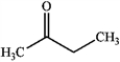
C)
D)

A)

B)

C)

D)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 43 في هذه المجموعة.
فتح الحزمة
k this deck
15
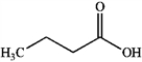
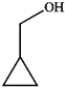
Which of the following compounds gives an infrared spectrum with peaks at 3300 cm−1 (strong, broad peak) and 1640 cm−1 (sharp, weak peak)?
A)
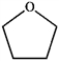
B)
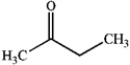
C)
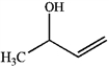
D)
A)

B)

C)

D)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 43 في هذه المجموعة.
فتح الحزمة
k this deck
16
What is the vertical axis of a mass spectrum?
A) mass
B) energy
C) abundance
D) field strength
A) mass
B) energy
C) abundance
D) field strength

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 43 في هذه المجموعة.
فتح الحزمة
k this deck
17
Examining the infrared spectrum of a compound allows us to:
A) determine the types of functional groups present in the compound
B) determine the carbon-hydrogen framework of the compound
C) determine the molecular weight of the compound
D) determine the nature of the conjugated pi electron system in the compound
A) determine the types of functional groups present in the compound
B) determine the carbon-hydrogen framework of the compound
C) determine the molecular weight of the compound
D) determine the nature of the conjugated pi electron system in the compound

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 43 في هذه المجموعة.
فتح الحزمة
k this deck
18
Which of the following bonds gives rise to a strong absorbance near 1700 cm−1 in the infrared spectrum?
A) C=O
B) C−O
C) C=C
D) C−C
A) C=O
B) C−O
C) C=C
D) C−C

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 43 في هذه المجموعة.
فتح الحزمة
k this deck
19
Refer to instructions. M+ at m/z = 216
A) C6H13OCl
B) C4H8Br2
C) C10H16
D) C9H12O
A) C6H13OCl
B) C4H8Br2
C) C10H16
D) C9H12O

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 43 في هذه المجموعة.
فتح الحزمة
k this deck
20
Which of the following compounds gives an infrared spectrum with peaks at 3000−3500 cm−1 and ˜1750 cm−1?
A)
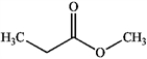
B)
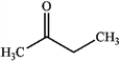
C)
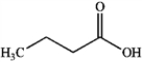
D)

A)

B)

C)

D)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 43 في هذه المجموعة.
فتح الحزمة
k this deck
21
Below is the mass spectrum of an unknown hydrocarbon. In addition, this hydrocarbon shows characteristic absorption at 2100 cm−1 in its IR spectrum. Give the structure of this unknown. 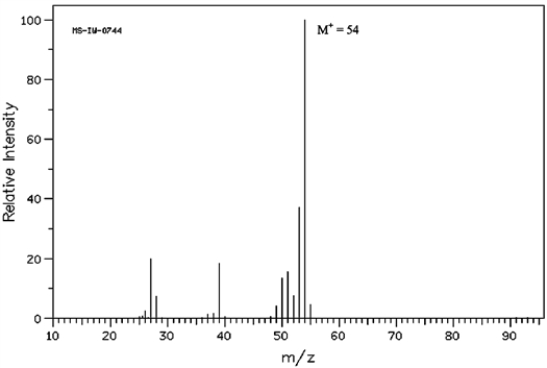 (Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/)
(Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/)
 (Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/)
(Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/)
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 43 في هذه المجموعة.
فتح الحزمة
k this deck
22
Assume you are carrying out the conversion of 1-bromobutane to butan-1-ol. How could you use IR spectroscopy to determine when the reaction is complete? 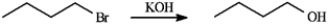
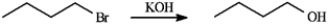

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 43 في هذه المجموعة.
فتح الحزمة
k this deck
23
What region of the IR spectrum would be the most useful in distinguishing between the following two compounds? 
A) Above 2850 cm−1
B) Between 2850 and 2000 cm−1
C) Between 2000 and 1500 cm−1
D) Below 1500 cm−1

A) Above 2850 cm−1
B) Between 2850 and 2000 cm−1
C) Between 2000 and 1500 cm−1
D) Below 1500 cm−1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 43 في هذه المجموعة.
فتح الحزمة
k this deck
24
Refer to the mass spectrum of 2-methylbutane shown below to answer the following question(s). 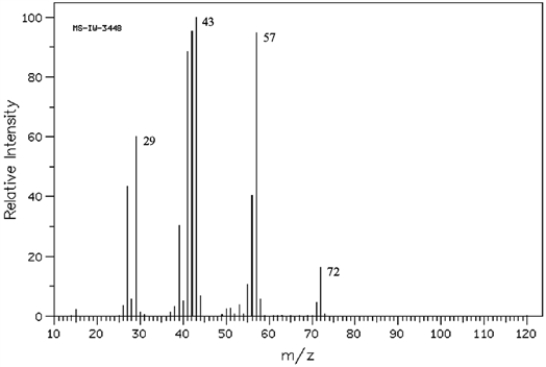 (Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/)
(Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/)
Refer to instructions. What peak represents M+?
 (Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/)
(Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/)Refer to instructions. What peak represents M+?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 43 في هذه المجموعة.
فتح الحزمة
k this deck
25
Which type of spectroscopy (IR, UV, or MS) will best distinguish between the pair of compounds below? Give a brief reason. 


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 43 في هذه المجموعة.
فتح الحزمة
k this deck
26
At what approximate positions might the compound below show IR absorptions? 


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 43 في هذه المجموعة.
فتح الحزمة
k this deck
27
Match each of the following groups of bond-types to the region of the infrared spectrum in which their absorptions occur.
C−C, C−O, C−N, and C−X single-bond vibrations.
A)4000 to 2500 cm−1
B)2500 to 2000 cm−1
C)2000 to 1500 cm−1
D)below 1500 cm−1
C−C, C−O, C−N, and C−X single-bond vibrations.
A)4000 to 2500 cm−1
B)2500 to 2000 cm−1
C)2000 to 1500 cm−1
D)below 1500 cm−1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 43 في هذه المجموعة.
فتح الحزمة
k this deck
28
Arrange the following in order of increasing energy.
A. 3842 nm B. 2870 cm−1 C. 2.91 × 1016 Hz D. 1.99 × 10−18 J
A. 3842 nm B. 2870 cm−1 C. 2.91 × 1016 Hz D. 1.99 × 10−18 J

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 43 في هذه المجموعة.
فتح الحزمة
k this deck
29
Refer to the mass spectrum of 2-methylbutane shown below to answer the following question(s).  (Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/)
(Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/)
Refer to instructions. What peak represents the base peak?
 (Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/)
(Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/)Refer to instructions. What peak represents the base peak?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 43 في هذه المجموعة.
فتح الحزمة
k this deck
30
Loratidine is the active ingredient in the antihistamine Claritin. Mass spectral analysis of loratidine shows M+ at m/z = 382. Loratidine is known to contain nitrogen. What is the minimum number of nitrogens in loratidine?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 43 في هذه المجموعة.
فتح الحزمة
k this deck
31
What are the masses of the charged fragments produced by alpha cleavage of the following? 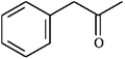


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 43 في هذه المجموعة.
فتح الحزمة
k this deck
32
Refer to the mass spectrum of 2-methylbutane shown below to answer the following question(s).  (Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/)
(Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/)
Refer to instructions. Propose structures for fragment ions at m/z = 57, 43, and 29.
 (Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/)
(Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/)Refer to instructions. Propose structures for fragment ions at m/z = 57, 43, and 29.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 43 في هذه المجموعة.
فتح الحزمة
k this deck
33
A 3.42 × 10−5 M solution of dibenzalacetone in ethanol produced an absorbance of 0.753 in a 1.00 cm cell. Based on this data, what is ε for this compound?
A) 2.57 × 105
B) 2.20 × 104
C) 4.54 × 10−5
D) 2.92 × 104
A) 2.57 × 105
B) 2.20 × 104
C) 4.54 × 10−5
D) 2.92 × 104

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 43 في هذه المجموعة.
فتح الحزمة
k this deck
34
Which of the following compounds would have the longest λmax in the UV region of the electromagnetic spectrum? A 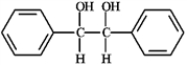 B
B 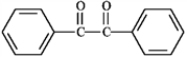 C
C 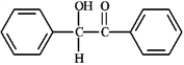
A) A
B) B
C) C
D) All would have the same λmax.
 B
B 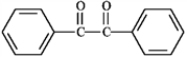 C
C 
A) A
B) B
C) C
D) All would have the same λmax.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 43 في هذه المجموعة.
فتح الحزمة
k this deck
35
The source of absorbance of light at a wavelength of 6600 nm would be:
A) π → π* electron transitions
B) bond stretching
C) in-plane bending
D) out-of-plane bending
E) b, c, d
A) π → π* electron transitions
B) bond stretching
C) in-plane bending
D) out-of-plane bending
E) b, c, d

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 43 في هذه المجموعة.
فتح الحزمة
k this deck
36
What are the masses of the charged fragments produced by the dehydration of the following compound? 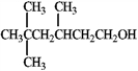
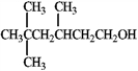

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 43 في هذه المجموعة.
فتح الحزمة
k this deck
37
Cyclohexene and hex-2-yne both have the molecular formula, C6H10.
a)How would you use infrared spectroscopy to distinguish between the two compounds?
b)How could the mass spectrum be used to distinguish between the two compounds?
a)How would you use infrared spectroscopy to distinguish between the two compounds?
b)How could the mass spectrum be used to distinguish between the two compounds?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 43 في هذه المجموعة.
فتح الحزمة
k this deck
38
Consider the compound below: 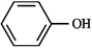 a)At what approximate positions might the compound show IR absorptions?
a)At what approximate positions might the compound show IR absorptions?
b)How would this spectrum differ from that of cyclohexanol?
 a)At what approximate positions might the compound show IR absorptions?
a)At what approximate positions might the compound show IR absorptions?b)How would this spectrum differ from that of cyclohexanol?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 43 في هذه المجموعة.
فتح الحزمة
k this deck
39
Which of the following peaks is not prominent in the mass spectrum of 2-methylbutan-2-ol?
A) M+ − 15
B) M+ − 18
C) M+ − 21
D) M+ − 29
A) M+ − 15
B) M+ − 18
C) M+ − 21
D) M+ − 29

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 43 في هذه المجموعة.
فتح الحزمة
k this deck
40
Circle any of the following compounds that would be a candidate to produce a UV absorption spectrum. 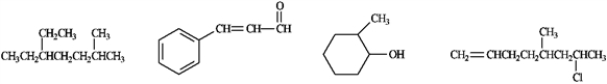


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 43 في هذه المجموعة.
فتح الحزمة
k this deck
41
Match each of the following groups of bond-types to the region of the infrared spectrum in which their absorptions occur.
N−H, C−H, and O−H stretching and bending motions.
A)4000 to 2500 cm−1
B)2500 to 2000 cm−1
C)2000 to 1500 cm−1
D)below 1500 cm−1
N−H, C−H, and O−H stretching and bending motions.
A)4000 to 2500 cm−1
B)2500 to 2000 cm−1
C)2000 to 1500 cm−1
D)below 1500 cm−1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 43 في هذه المجموعة.
فتح الحزمة
k this deck
42
Match each of the following groups of bond-types to the region of the infrared spectrum in which their absorptions occur.
C=O, C=N, and C=C bond absorptions.
A)4000 to 2500 cm−1
B)2500 to 2000 cm−1
C)2000 to 1500 cm−1
D)below 1500 cm−1
C=O, C=N, and C=C bond absorptions.
A)4000 to 2500 cm−1
B)2500 to 2000 cm−1
C)2000 to 1500 cm−1
D)below 1500 cm−1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 43 في هذه المجموعة.
فتح الحزمة
k this deck
43
Match each of the following groups of bond-types to the region of the infrared spectrum in which their absorptions occur.
triple bond stretching vibrations.
A)4000 to 2500 cm−1
B)2500 to 2000 cm−1
C)2000 to 1500 cm−1
D)below 1500 cm−1
triple bond stretching vibrations.
A)4000 to 2500 cm−1
B)2500 to 2000 cm−1
C)2000 to 1500 cm−1
D)below 1500 cm−1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 43 في هذه المجموعة.
فتح الحزمة
k this deck








