Deck 1: Structure and Bonding
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/29
العب
ملء الشاشة (f)
Deck 1: Structure and Bonding
1
The molecular formula C2H4O can be converted into three-line bond (Kekulé) structures that are consistent with valence rules. Which one of the following Kekulé structures is not consistent with valence rules?
A)
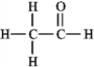
B)
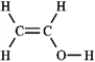
C)
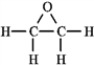
D)
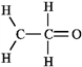
A)

B)

C)

D)


2
Which of the following statements is not true?
A) The carbon−carbon single bond of an alkane is weaker than the carbon−carbon triple bond of an alkyne.
B) The carbon−carbon triple bond of an alkyne is shorter than the carbon−carbon double bond of an alkene.
C) The carbon−carbon triple bond of an alkyne is exactly three times as strong as a carbon−carbon single bond of an alkane.
D) The carbon−carbon single bond of an alkane is longer than the carbon−carbon triple bond of an alkyne.
A) The carbon−carbon single bond of an alkane is weaker than the carbon−carbon triple bond of an alkyne.
B) The carbon−carbon triple bond of an alkyne is shorter than the carbon−carbon double bond of an alkene.
C) The carbon−carbon triple bond of an alkyne is exactly three times as strong as a carbon−carbon single bond of an alkane.
D) The carbon−carbon single bond of an alkane is longer than the carbon−carbon triple bond of an alkyne.
The carbon−carbon triple bond of an alkyne is exactly three times as strong as a carbon−carbon single bond of an alkane.
3
Consider the formation of an sp2 hybrid orbital. Which of the following is true?
A) Four equivalent hybrid orbitals are produced.
B) One s and one p atomic orbital are involved.
C) One p atomic orbital remains unhybridized.
D) The hybrid orbitals produced can form π bonds.
E) none of these
A) Four equivalent hybrid orbitals are produced.
B) One s and one p atomic orbital are involved.
C) One p atomic orbital remains unhybridized.
D) The hybrid orbitals produced can form π bonds.
E) none of these
One p atomic orbital remains unhybridized.
4
Propose a structure for a molecule that meets the following description.
Refer to instructions. Contains only two sp3 hybridized carbons and two sp hybridized carbons.
Refer to instructions. Contains only two sp3 hybridized carbons and two sp hybridized carbons.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 29 في هذه المجموعة.
فتح الحزمة
k this deck
5
How many electrons are there in the valence shell of the carbon atom of a methyl anion, CH3−?
A) 5
B) 6
C) 7
D) 8
A) 5
B) 6
C) 7
D) 8

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 29 في هذه المجموعة.
فتح الحزمة
k this deck
6
Convert the skeletal drawing of the pharmaceutical Vioxx into a molecular formula. 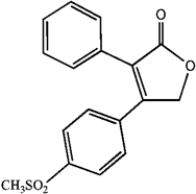


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 29 في هذه المجموعة.
فتح الحزمة
k this deck
7
In drawing the Lewis structure for an organic compound, the carbon atoms should always be shown with
A) lone pairs of electrons.
B) four single bonds.
C) eight total electrons.
D) a positive charge.
E) none of these
A) lone pairs of electrons.
B) four single bonds.
C) eight total electrons.
D) a positive charge.
E) none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 29 في هذه المجموعة.
فتح الحزمة
k this deck
8
Propose a structure for a molecule that meets the following description.
Refer to instructions. Contains only one sp3 hybridized carbon and two sp2 hybridized carbons.
Refer to instructions. Contains only one sp3 hybridized carbon and two sp2 hybridized carbons.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 29 في هذه المجموعة.
فتح الحزمة
k this deck
9
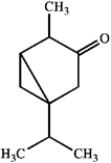
Specify the hybridization of each carbon atom of limonene, a natural product present in citrus fruits, and thujone, which is derived from wormwood, a traditional component of the notorious liquor, Absinthe. 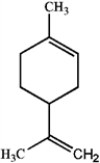
 limonene
limonene
thujone

 limonene
limonenethujone

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 29 في هذه المجموعة.
فتح الحزمة
k this deck
10
Determine the hybridization for the indicated atoms in each structure below. 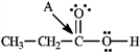
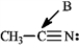
Refer to instructions. The hybridization of carbon atom B is _____.
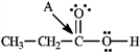
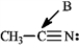
Refer to instructions. The hybridization of carbon atom B is _____.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 29 في هذه المجموعة.
فتح الحزمة
k this deck
11
Write valid Lewis (electron-dot) structures for each formula below. Show all electrons as dots and show all nonbonding electrons.
Write:
CH3CH2OH ethanol
Write:
CH3CH2OH ethanol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 29 في هذه المجموعة.
فتح الحزمة
k this deck
12
Determine the hybridization for the indicated atoms in each structure below. 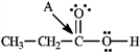
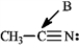
Refer to instructions. The hybridization of carbon atom A is _____.
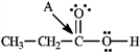
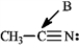
Refer to instructions. The hybridization of carbon atom A is _____.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 29 في هذه المجموعة.
فتح الحزمة
k this deck
13
How many nonbonding electron pairs are in the structure shown below? 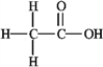
A) 2
B) 4
C) 6
D) 8
E) none of these

A) 2
B) 4
C) 6
D) 8
E) none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 29 في هذه المجموعة.
فتح الحزمة
k this deck
14
Which of the following best represents the shape of a sp3 hybrid orbital of carbon? A  B
B  C
C  D
D 
A) A
B) B
C) C
D) D
 B
B  C
C  D
D 
A) A
B) B
C) C
D) D

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 29 في هذه المجموعة.
فتح الحزمة
k this deck
15
Which of the following best represents the shape of a 2p atomic orbital of carbon? A  B
B  C
C  D
D 
A) A
B) B
C) C
D) D
 B
B  C
C  D
D 
A) A
B) B
C) C
D) D

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 29 في هذه المجموعة.
فتح الحزمة
k this deck
16
How many total valence electrons are represented in the following electron configuration? 1s22s22px2 2py2 2pz1 or 1s22s22p5
A) 1
B) 3
C) 5
D) 7
E) 9
A) 1
B) 3
C) 5
D) 7
E) 9

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 29 في هذه المجموعة.
فتح الحزمة
k this deck
17
Covalent bonding
A) involves a transfer of electrons from one atom to another.
B) occurs when atoms share all their valence electrons.
C) occurs when unpaired valence electrons are shared between atoms.
D) occurs when nonvalence electrons are shared between atoms.
E) none of these
A) involves a transfer of electrons from one atom to another.
B) occurs when atoms share all their valence electrons.
C) occurs when unpaired valence electrons are shared between atoms.
D) occurs when nonvalence electrons are shared between atoms.
E) none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 29 في هذه المجموعة.
فتح الحزمة
k this deck
18
According to atomic theory:
A) the nucleus is positively charged.
B) the nucleus contains both charged and uncharged particles.
C) the electrons contribute very little to the total mass of the atom.
D) the electrons are located in the atomic space outside the nucleus.
E) all of these
A) the nucleus is positively charged.
B) the nucleus contains both charged and uncharged particles.
C) the electrons contribute very little to the total mass of the atom.
D) the electrons are located in the atomic space outside the nucleus.
E) all of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 29 في هذه المجموعة.
فتح الحزمة
k this deck
19
Draw all the lone pairs (nonbonding valence electrons) on the structure of phosgene, a poisonous gas once used as a chemical warfare agent. 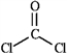


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 29 في هذه المجموعة.
فتح الحزمة
k this deck
20
Write valid Lewis (electron-dot) structures for each formula below. Show all electrons as dots and show all nonbonding electrons.
The structure of urea is shown below. Fill in any nonbonding valence electrons that are missing from the line-bond structure.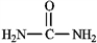
The structure of urea is shown below. Fill in any nonbonding valence electrons that are missing from the line-bond structure.
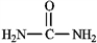

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 29 في هذه المجموعة.
فتح الحزمة
k this deck
21
Draw two possible isomers of C6H6 in which all the carbon atoms are sp2 hybridized.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 29 في هذه المجموعة.
فتح الحزمة
k this deck
22
Consider the two structures below to answer the following question.CH3CH2OH CH3OCH3
Refer to instructions. Which of the following correctly describes the structure of these compounds?
A) All carbon atoms are sp3 hybridized.
B) All of the bonds are sigma bonds.
C) Each oxygen atom has two nonbonding pairs of electrons.
D) The bond angle around each oxygen atom is ideally about 109.5°.
E) All of these
Refer to instructions. Which of the following correctly describes the structure of these compounds?
A) All carbon atoms are sp3 hybridized.
B) All of the bonds are sigma bonds.
C) Each oxygen atom has two nonbonding pairs of electrons.
D) The bond angle around each oxygen atom is ideally about 109.5°.
E) All of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 29 في هذه المجموعة.
فتح الحزمة
k this deck
23
Consider the two structures below to answer the following question.CH3CH2OH CH3OCH3
Which of the following statements is not true according to molecular orbital (MO) theory?
A) Antibonding orbitals are higher in energy than the corresponding bonding orbital.
B) The head-on overlap of an s and a p atomic orbital can produce a σ molecular orbital.
C) A π molecular orbital forms only from the combination of p atomic orbital wave functions.
D) The subtractive combination of atomic orbital wave functions produces a bonding molecular orbital.
Which of the following statements is not true according to molecular orbital (MO) theory?
A) Antibonding orbitals are higher in energy than the corresponding bonding orbital.
B) The head-on overlap of an s and a p atomic orbital can produce a σ molecular orbital.
C) A π molecular orbital forms only from the combination of p atomic orbital wave functions.
D) The subtractive combination of atomic orbital wave functions produces a bonding molecular orbital.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 29 في هذه المجموعة.
فتح الحزمة
k this deck
24
Draw the structure for CCl2F2 using solid, wedged, and dashed lines to show the tetrahedral geometry.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 29 في هذه المجموعة.
فتح الحزمة
k this deck
25
Draw a picture showing the orbitals involved in the π-bonds of cyclopenta-1,3-diene, a commonly encountered reagent in organic synthesis. 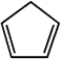


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 29 في هذه المجموعة.
فتح الحزمة
k this deck
26
Consider the two structures below to answer the following question.CH3CH2OH CH3OCH3
In the two structures shown below, what do the positions labeled with the arrow have in common?
A) the same type of hybridization on the carbon atom
B) the same geometry around the carbon atom
C) the same number of hydrogen atoms bonded to the carbon atom
D) both carbon atoms are involved in a π bond
In the two structures shown below, what do the positions labeled with the arrow have in common?

A) the same type of hybridization on the carbon atom
B) the same geometry around the carbon atom
C) the same number of hydrogen atoms bonded to the carbon atom
D) both carbon atoms are involved in a π bond

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 29 في هذه المجموعة.
فتح الحزمة
k this deck
27
Consider the two structures below to answer the following question.CH3CH2OH CH3OCH3
The molecular orbital shown below is most likely of what type?
A) σ bonding
B) σ antibonding
C) π bonding
D) π antibonding
The molecular orbital shown below is most likely of what type?

A) σ bonding
B) σ antibonding
C) π bonding
D) π antibonding

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 29 في هذه المجموعة.
فتح الحزمة
k this deck
28
Draw all possible structures of CFnClm where n and m vary from 0 to 4.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 29 في هذه المجموعة.
فتح الحزمة
k this deck
29
Consider the two structures below to answer the following question.CH3CH2OH CH3OCH3
What is the expected hybridization around the sulfur atom in diethyl sulfide? CH3CH2⎯S⎯CH2CH3
A) sp
B) sp2
C) sp3
D) The sulfur atom is not hybridized.
What is the expected hybridization around the sulfur atom in diethyl sulfide? CH3CH2⎯S⎯CH2CH3
A) sp
B) sp2
C) sp3
D) The sulfur atom is not hybridized.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 29 في هذه المجموعة.
فتح الحزمة
k this deck








