Deck 18: Ethers and Epoxides;thiols and Sulfides
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/33
العب
ملء الشاشة (f)
Deck 18: Ethers and Epoxides;thiols and Sulfides
1
Exhibit 18-3
Consider the data below to answer the following question(s).
Epoxides are synthesized industrially in one step by silver oxide air oxidation of ethylene and on a laboratory scale in one step by treating an alkene with m-chloroperoxybenzoic acid.An alternative two-step process converts alkenes to halohydrins,which are converted by treatment with base to epoxides.
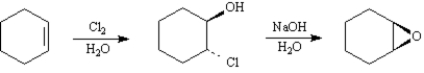
Refer to Exhibit 18-3.The synthesis of epoxides by base treatment of halohydrins is an example of an intramolecular:
A)SN1 reaction
B)hydrolysis reaction
C)dehydration reaction
D)Williamson ether synthesis
Consider the data below to answer the following question(s).
Epoxides are synthesized industrially in one step by silver oxide air oxidation of ethylene and on a laboratory scale in one step by treating an alkene with m-chloroperoxybenzoic acid.An alternative two-step process converts alkenes to halohydrins,which are converted by treatment with base to epoxides.

Refer to Exhibit 18-3.The synthesis of epoxides by base treatment of halohydrins is an example of an intramolecular:
A)SN1 reaction
B)hydrolysis reaction
C)dehydration reaction
D)Williamson ether synthesis
Williamson ether synthesis
2
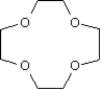
Draw: 12-crown-4
3
Draw: cyclopropyl ethyl sulfide
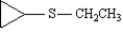
4
Diphenyl ether is inert to cleavage by HI or HBr.Explain. 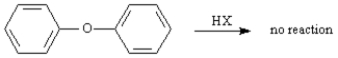


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
5
Exhibit 18-1
Consider the reaction below to answer the following question(s).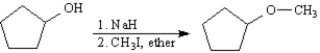
Refer to Exhibit 18-1.Alternatively,cyclopentyl methyl ether may be synthesized from cyclopentene.Outline a synthesis of cyclopentyl methyl ether from cyclopentene.
Consider the reaction below to answer the following question(s).
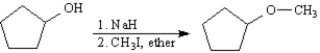
Refer to Exhibit 18-1.Alternatively,cyclopentyl methyl ether may be synthesized from cyclopentene.Outline a synthesis of cyclopentyl methyl ether from cyclopentene.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
6
When 1,2-epoxypropane is treated with sodium ethoxide in ethanol 1-ethoxy-2-propanol is the major product while only a trace amount of 2-ethoxy-1-propanol is produced.Explain these results based on the reaction mechanism. 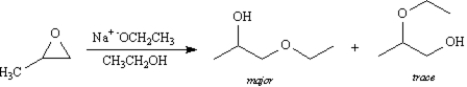


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
7
Exhibit 18-2
Consider the data below to answer the following question(s).
Acetals are a special class of ethers which have two ether linkages at a single carbon.Tetrahydropyranyl ethers are acetals that are easily cleaved with mild aqueous acids at room temperature to yield alcohols.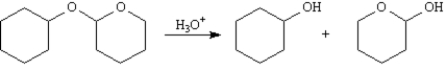
Refer to Exhibit 18-2.Draw arrows showing the electron flow for this reaction on the structures provided below.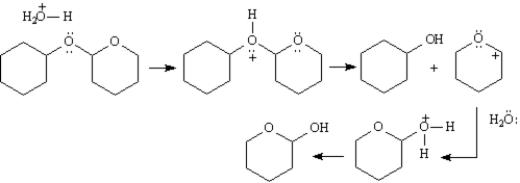
Consider the data below to answer the following question(s).
Acetals are a special class of ethers which have two ether linkages at a single carbon.Tetrahydropyranyl ethers are acetals that are easily cleaved with mild aqueous acids at room temperature to yield alcohols.

Refer to Exhibit 18-2.Draw arrows showing the electron flow for this reaction on the structures provided below.


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
8
Exhibit 18-1
Consider the reaction below to answer the following question(s).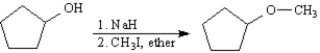
Refer to Exhibit 18-1.Write the complete stepwise mechanism for the reaction.Show all intermediate structures and all electron flow with arrows.
Consider the reaction below to answer the following question(s).
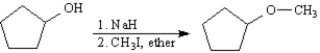
Refer to Exhibit 18-1.Write the complete stepwise mechanism for the reaction.Show all intermediate structures and all electron flow with arrows.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
9
Draw: allyl benzyl ether

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
10
Draw: 1-isopropoxycyclopentene

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
11
Exhibit 18-3
Consider the data below to answer the following question(s).
Epoxides are synthesized industrially in one step by silver oxide air oxidation of ethylene and on a laboratory scale in one step by treating an alkene with m-chloroperoxybenzoic acid.An alternative two-step process converts alkenes to halohydrins,which are converted by treatment with base to epoxides.

Refer to Exhibit 18-3.Show electron flow with arrows on the structures provided below for each step in the above transformation.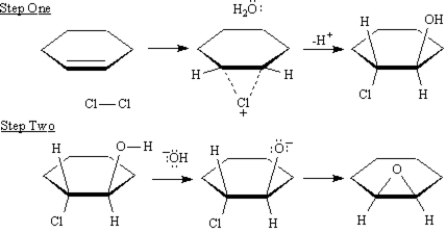
Consider the data below to answer the following question(s).
Epoxides are synthesized industrially in one step by silver oxide air oxidation of ethylene and on a laboratory scale in one step by treating an alkene with m-chloroperoxybenzoic acid.An alternative two-step process converts alkenes to halohydrins,which are converted by treatment with base to epoxides.

Refer to Exhibit 18-3.Show electron flow with arrows on the structures provided below for each step in the above transformation.


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
12
Name: 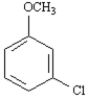


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
13
Name: 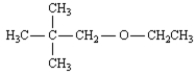


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
14
Exhibit 18-1
Consider the reaction below to answer the following question(s).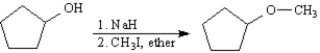
Refer to Exhibit 18-1.Mechanistically,the Williamson ether synthesis outlined above is:
A)an E1 process
B)an SN1 process
C)an E2 process
D)an SN2 process
Consider the reaction below to answer the following question(s).
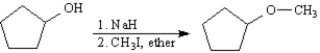
Refer to Exhibit 18-1.Mechanistically,the Williamson ether synthesis outlined above is:
A)an E1 process
B)an SN1 process
C)an E2 process
D)an SN2 process

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
15
Exhibit 18-2
Consider the data below to answer the following question(s).
Acetals are a special class of ethers which have two ether linkages at a single carbon.Tetrahydropyranyl ethers are acetals that are easily cleaved with mild aqueous acids at room temperature to yield alcohols.
Refer to Exhibit 18-2.This ether cleavage occurs so easily under such mild conditions because the carbocation intermediate in step two is very stable.Explain why this carbocation is stabilized.
Consider the data below to answer the following question(s).
Acetals are a special class of ethers which have two ether linkages at a single carbon.Tetrahydropyranyl ethers are acetals that are easily cleaved with mild aqueous acids at room temperature to yield alcohols.

Refer to Exhibit 18-2.This ether cleavage occurs so easily under such mild conditions because the carbocation intermediate in step two is very stable.Explain why this carbocation is stabilized.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
16
Draw: tetrahydrofuran

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
17
Write the complete stepwise mechanism for the reaction below.Show all electron flow with arrows and draw all intermediate structures. 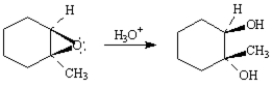


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
18
Name: 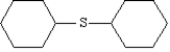
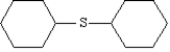

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
19
Draw: 3-methyl-1-butanethiol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
20
Draw: diethyl ether

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
21
Propose a synthesis for the following compound using benzene or toluene and any other reagents necessary.Show all major intermediate compounds that would probably be isolated during the course of your synthesis. 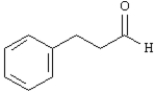


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
22
The following is the proton NMR of a symmetrical alkyl ether.Which peak is most likely due to the protons adjacent to the ether oxygen atom? 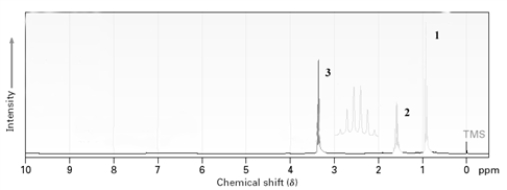
A)1
B)2
C)3

A)1
B)2
C)3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
23
Choose the best reagent for carrying out the following reactions from the list below.Place the letter of the reagent(s) in the box over the reaction arrow.Use only one letter per box. 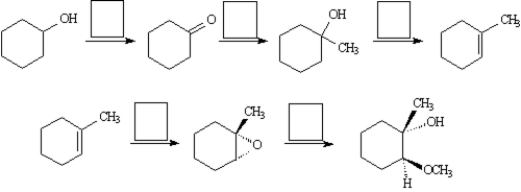 A.
A.
NaH,then CH3I
B.
NaOCH3,CH3OH
C.
m-ClC6H4CO3H
D.
CH3MgBr in ether,then H3O+
E.
warm H2SO4/H2O
F.
Hg(O2CCF3)2,CH3OH
G.
H2/Pd
H.
PCC,CH2Cl2
I.
Cl2,H2O
J.
LiAlH4 in ether,then H3O+
 A.
A.NaH,then CH3I
B.
NaOCH3,CH3OH
C.
m-ClC6H4CO3H
D.
CH3MgBr in ether,then H3O+
E.
warm H2SO4/H2O
F.
Hg(O2CCF3)2,CH3OH
G.
H2/Pd
H.
PCC,CH2Cl2
I.
Cl2,H2O
J.
LiAlH4 in ether,then H3O+

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
24
Complete the synthetic sequence below by drawing the structures of the reaction in the boxes provided. 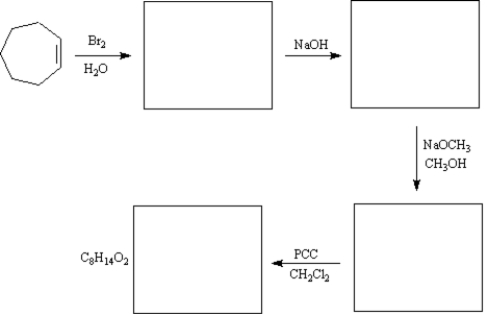


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
25
Name the following compound.Atoms other than carbon and hydrogen are labeled. 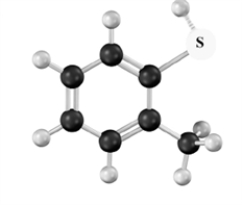


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
26
Name the compound shown below.Atoms other than carbon and hydrogen are labeled. 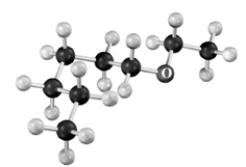


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
27
Show how the ether below could be prepared from toluene and any other necessary reagents.Show all reagents and all intermediate structures. 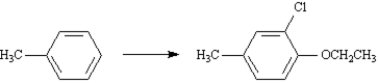


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
28
Which of the following will undergo rearrangement upon heating?
A)2-butenyl phenyl ether
B)1-propenyl 2-propenyl ether
C)2-pentenyl phenyl ether
D)All will undergo rearrangement.
A)2-butenyl phenyl ether
B)1-propenyl 2-propenyl ether
C)2-pentenyl phenyl ether
D)All will undergo rearrangement.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
29
Predict the product of the following reaction. 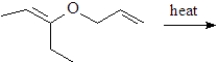


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
30
Although not always evident in an IR spectrum,which peak in the IR spectrum below might be due to the C-O stretch of an ether? 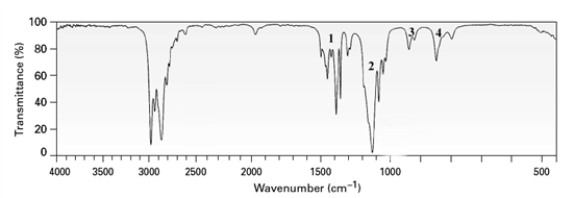
A)1
B)2
C)3
D)4

A)1
B)2
C)3
D)4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
31
Consider the following species.Atoms other than carbon and hydrogen are labeled. 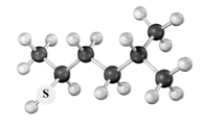 Which of the following would be produced upon treatment with I2?
Which of the following would be produced upon treatment with I2?
A)
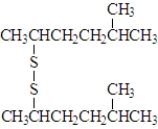
B)
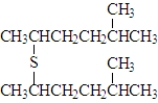
C)
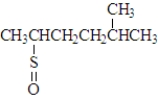
D)
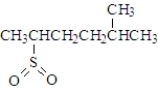
 Which of the following would be produced upon treatment with I2?
Which of the following would be produced upon treatment with I2?A)
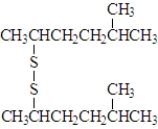
B)
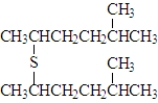
C)
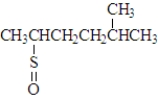
D)
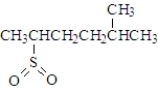

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
32
What is the product formed upon treatment of the following substance with hydrogen peroxide at room temperature,followed by treatment with a peroxyacid? Atoms other than carbon and hydrogen are labeled. 
A)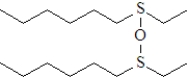
B)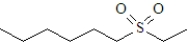
C)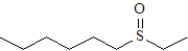
D)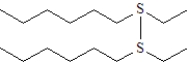

A)

B)

C)

D)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
33
Draw the structure of the ether that could be used to produce the following compound. 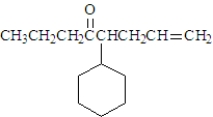


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck








