Deck 23: Carbonyl Condensation Reactions
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/36
العب
ملء الشاشة (f)
Deck 23: Carbonyl Condensation Reactions
1
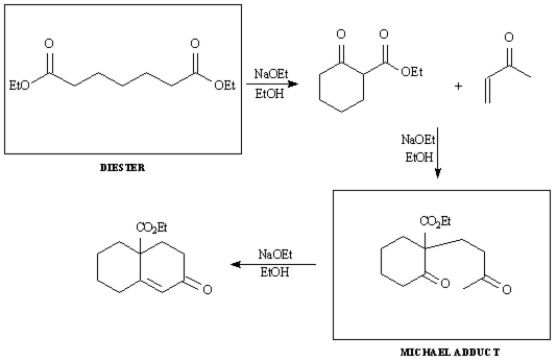
Provide the indicated starting material and intermediate in the synthetic sequence below involving a Dieckmann cyclization,followed by a Robinson annulation. 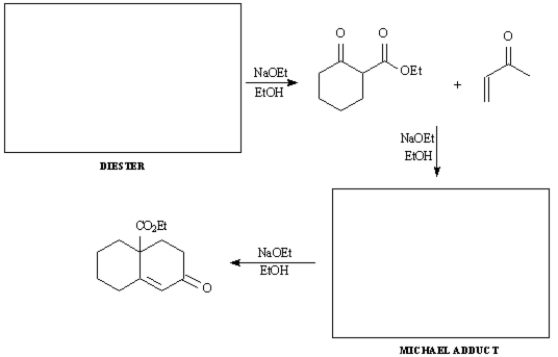

2
Exhibit 23-10
Consider the reaction below to answer the following question(s).
The Stork enamine reaction is a variation on the Michael reaction which utilizes an enamine nucleophile.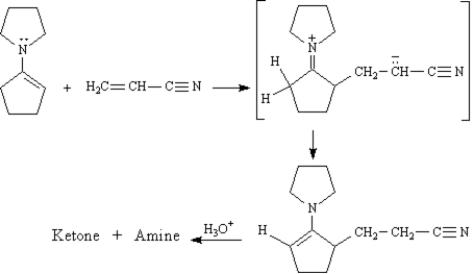
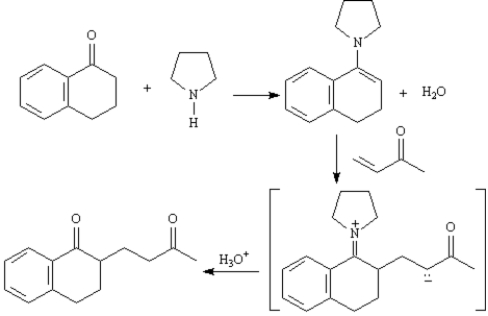
Refer to Exhibit 23-10.Show how you might use a Stork enamine reaction to prepare the following compound.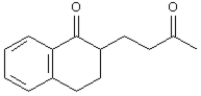
Consider the reaction below to answer the following question(s).
The Stork enamine reaction is a variation on the Michael reaction which utilizes an enamine nucleophile.

Refer to Exhibit 23-10.Show how you might use a Stork enamine reaction to prepare the following compound.

3
Exhibit 23-2
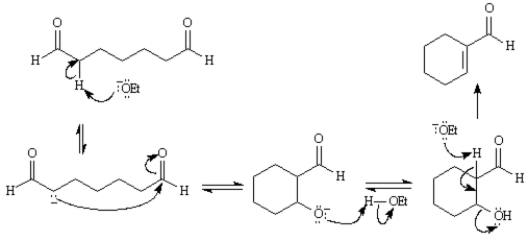
Consider the reaction below to answer the following question(s):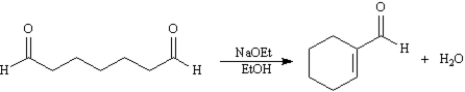
Refer to Exhibit 23-2.Write the complete stepwise mechanism for the reaction above.Show all intermediate structures and all electron flow with arrows.
Consider the reaction below to answer the following question(s):
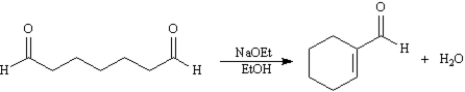
Refer to Exhibit 23-2.Write the complete stepwise mechanism for the reaction above.Show all intermediate structures and all electron flow with arrows.
4
Show how you might use a Robinson annulation reaction to synthesize the following compound.Draw the structures of both reactants and the structure of the intermediate Michael addition product. 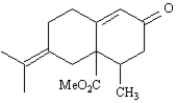


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 36 في هذه المجموعة.
فتح الحزمة
k this deck
5
Exhibit 23-5
Consider the reaction below to answer the following question(s).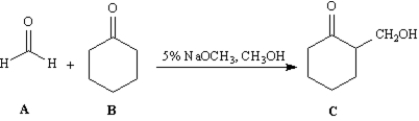
Refer to Exhibit 23-5.Which carbonyl compound functions as the electrophile in this reaction?
Consider the reaction below to answer the following question(s).
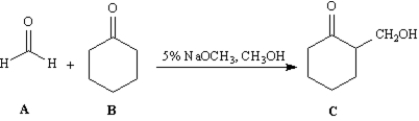
Refer to Exhibit 23-5.Which carbonyl compound functions as the electrophile in this reaction?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 36 في هذه المجموعة.
فتح الحزمة
k this deck
6
Ethyl acetate can be prepared from ethanol as the only organic starting material.Show all reagents and structures for all intermediates in this preparation.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 36 في هذه المجموعة.
فتح الحزمة
k this deck
7
Exhibit 23-3
Consider the data below to answer the following question(s):
The Friedlander Quinoline Synthesis,first reported in 1882,is the base-catalyzed condensation of 2-aminobenzaldehydes with ketones to form quinoline derivatives.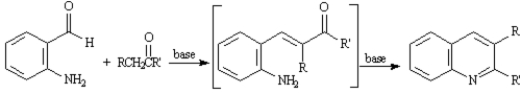
Refer to Exhibit 23-3.The second step of the Friedlander Quinoline Synthesis is a nucleophilic addition of a primary amine to a ketone yielding an imine.Write the complete stepwise mechanism for this imine forming reaction.Show all electron flow with arrows and show all intermediate structures.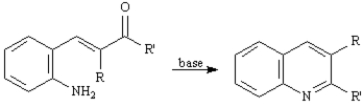
Consider the data below to answer the following question(s):
The Friedlander Quinoline Synthesis,first reported in 1882,is the base-catalyzed condensation of 2-aminobenzaldehydes with ketones to form quinoline derivatives.

Refer to Exhibit 23-3.The second step of the Friedlander Quinoline Synthesis is a nucleophilic addition of a primary amine to a ketone yielding an imine.Write the complete stepwise mechanism for this imine forming reaction.Show all electron flow with arrows and show all intermediate structures.


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 36 في هذه المجموعة.
فتح الحزمة
k this deck
8
Exhibit 23-5
Consider the reaction below to answer the following question(s).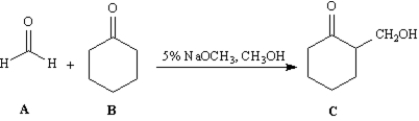
Refer to Exhibit 23-5.This reaction is an example of:
A)a mixed Claisen condensation.
B)a Dieckman condensation.
C)a Michael reaction.
D)a mixed aldol reaction.
Consider the reaction below to answer the following question(s).
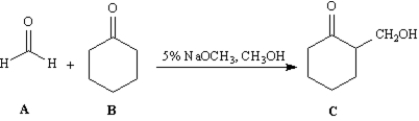
Refer to Exhibit 23-5.This reaction is an example of:
A)a mixed Claisen condensation.
B)a Dieckman condensation.
C)a Michael reaction.
D)a mixed aldol reaction.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 36 في هذه المجموعة.
فتح الحزمة
k this deck
9
Exhibit 23-2
Consider the reaction below to answer the following question(s):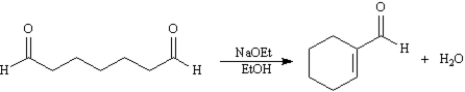
Refer to Exhibit 23-2.This reaction is an example of:
A)an intramolecular Claisen condensation
B)an intramolecular aldol condensation
C)a Robinson annulation
D)a Michael reaction
Consider the reaction below to answer the following question(s):
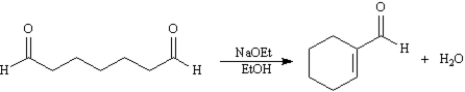
Refer to Exhibit 23-2.This reaction is an example of:
A)an intramolecular Claisen condensation
B)an intramolecular aldol condensation
C)a Robinson annulation
D)a Michael reaction

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 36 في هذه المجموعة.
فتح الحزمة
k this deck
10
Exhibit 23-2
Consider the reaction below to answer the following question(s):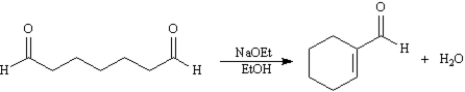
Refer to Exhibit 23-2.The product of this reaction is:
A)a β,γ-unsaturated aldehyde
B)an α,β-unsaturated ketone
C)an α,β-unsaturated aldehyde
D)an enol
Consider the reaction below to answer the following question(s):
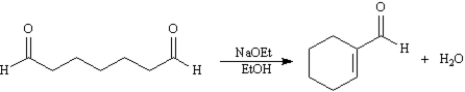
Refer to Exhibit 23-2.The product of this reaction is:
A)a β,γ-unsaturated aldehyde
B)an α,β-unsaturated ketone
C)an α,β-unsaturated aldehyde
D)an enol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 36 في هذه المجموعة.
فتح الحزمة
k this deck
11
In the alkylation of cyclohexanone,better yields are obtained by first reacting cyclohexanone with an equivalent of lithium diisopropylamide in THF and then adding the alkyl halide,rather than mixing cyclohexanone,alkyl halide,and a catalytic amount of sodium ethoxide in ethanol.Explain this observation by pointing out what the problems with the second reaction conditions might be and how the first set of reaction conditions help alleviate the problems.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 36 في هذه المجموعة.
فتح الحزمة
k this deck
12
Exhibit 23-10
Consider the reaction below to answer the following question(s).
The Stork enamine reaction is a variation on the Michael reaction which utilizes an enamine nucleophile.
Refer to Exhibit 23-10.Draw the structures of the Ketone + Amine products of this reaction.
Consider the reaction below to answer the following question(s).
The Stork enamine reaction is a variation on the Michael reaction which utilizes an enamine nucleophile.

Refer to Exhibit 23-10.Draw the structures of the Ketone + Amine products of this reaction.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 36 في هذه المجموعة.
فتح الحزمة
k this deck
13
Oxaloacetic acid is an important intermediate in the biosynthesis of citric acid.Synthesize oxaloacetic acid using a mixed Claisen condensation as a key carbon-carbon bond forming reaction. 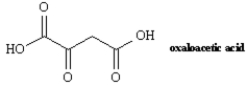


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 36 في هذه المجموعة.
فتح الحزمة
k this deck
14
Exhibit 23-7
Consider the reaction below to answer the following question(s):
Acetoacetic ester can be prepared by the Claisen self-condensation reaction of ethyl acetate.
Refer to Exhibit 23-7.Write the complete stepwise mechanism for this reaction.Show all electron flow with arrows and draw all intermediate structures.
Consider the reaction below to answer the following question(s):
Acetoacetic ester can be prepared by the Claisen self-condensation reaction of ethyl acetate.

Refer to Exhibit 23-7.Write the complete stepwise mechanism for this reaction.Show all electron flow with arrows and draw all intermediate structures.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 36 في هذه المجموعة.
فتح الحزمة
k this deck
15
Give the structures of the ester precursors for the following Claisen condensation product and formulate the reaction. 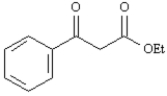


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 36 في هذه المجموعة.
فتح الحزمة
k this deck
16
Exhibit 23-10
Consider the reaction below to answer the following question(s).
The Stork enamine reaction is a variation on the Michael reaction which utilizes an enamine nucleophile.
Refer to Exhibit 23-10.On the structures above,draw arrows indicating electron flow in each step of this reaction.
Consider the reaction below to answer the following question(s).
The Stork enamine reaction is a variation on the Michael reaction which utilizes an enamine nucleophile.

Refer to Exhibit 23-10.On the structures above,draw arrows indicating electron flow in each step of this reaction.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 36 في هذه المجموعة.
فتح الحزمة
k this deck
17
Exhibit 23-5
Consider the reaction below to answer the following question(s).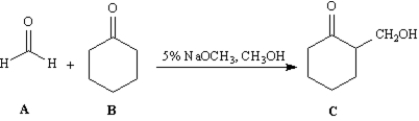
Refer to Exhibit 23-5.Draw the structure of the enolate ion that is generated during the course of this reaction.
Consider the reaction below to answer the following question(s).
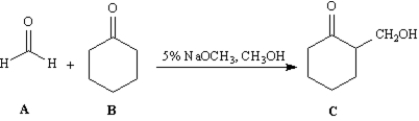
Refer to Exhibit 23-5.Draw the structure of the enolate ion that is generated during the course of this reaction.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 36 في هذه المجموعة.
فتح الحزمة
k this deck
18
Exhibit 23-8
Consider the compound 2-methyl-2-carboethoxycyclopentanone,whose structure is shown below,to answer the following question(s).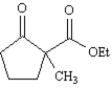
Refer to Exhibit 23-8.When 2-methyl-2-carboethoxycyclopentanone is treated with sodium ethoxide in ethanol solution followed by a mild aqueous acid work-up,5-methyl-2-carboethoxycyclopentanone is isolated as the major product.This reaction proceeds by a reverse Claisen condensation mechanism followed by a recyclization.On the structures provided below,show electron flow with arrows in this interesting reaction.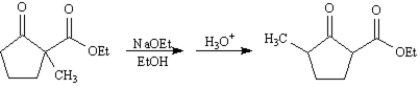
Consider the compound 2-methyl-2-carboethoxycyclopentanone,whose structure is shown below,to answer the following question(s).

Refer to Exhibit 23-8.When 2-methyl-2-carboethoxycyclopentanone is treated with sodium ethoxide in ethanol solution followed by a mild aqueous acid work-up,5-methyl-2-carboethoxycyclopentanone is isolated as the major product.This reaction proceeds by a reverse Claisen condensation mechanism followed by a recyclization.On the structures provided below,show electron flow with arrows in this interesting reaction.


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 36 في هذه المجموعة.
فتح الحزمة
k this deck
19
Exhibit 23-8
Consider the compound 2-methyl-2-carboethoxycyclopentanone,whose structure is shown below,to answer the following question(s).
Refer to Exhibit 23-8.Formulate a synthesis of 2-methyl-2-carboethoxycyclopentanone starting with acyclic precursors using a Dieckmann cyclization as a key carbon-carbon bond forming step.Show all reagents and all intermediate structures.
Consider the compound 2-methyl-2-carboethoxycyclopentanone,whose structure is shown below,to answer the following question(s).

Refer to Exhibit 23-8.Formulate a synthesis of 2-methyl-2-carboethoxycyclopentanone starting with acyclic precursors using a Dieckmann cyclization as a key carbon-carbon bond forming step.Show all reagents and all intermediate structures.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 36 في هذه المجموعة.
فتح الحزمة
k this deck
20
Exhibit 23-3
Consider the data below to answer the following question(s):
The Friedlander Quinoline Synthesis,first reported in 1882,is the base-catalyzed condensation of 2-aminobenzaldehydes with ketones to form quinoline derivatives.
Refer to Exhibit 23-3.The first step of the Friedlander Quinoline Synthesis is a mixed aldol condensation.Write the complete stepwise mechanism for this reaction.
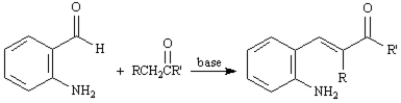
Consider the data below to answer the following question(s):
The Friedlander Quinoline Synthesis,first reported in 1882,is the base-catalyzed condensation of 2-aminobenzaldehydes with ketones to form quinoline derivatives.

Refer to Exhibit 23-3.The first step of the Friedlander Quinoline Synthesis is a mixed aldol condensation.Write the complete stepwise mechanism for this reaction.


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 36 في هذه المجموعة.
فتح الحزمة
k this deck
21
Draw the general mechanism of a carbonyl condensation reaction between two molecules of acetaldehyde.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 36 في هذه المجموعة.
فتح الحزمة
k this deck
22
What is the structure of the enamine produced in the reaction of cyclohexanone with piperidine? See structure below. 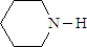
A)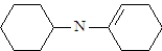
B)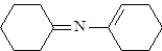
C)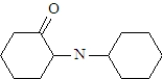
D)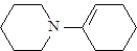
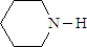
A)
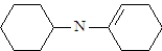
B)
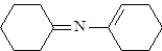
C)

D)
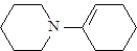

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 36 في هذه المجموعة.
فتح الحزمة
k this deck
23
Which of the following when reacted with butanal would be the most likely to produce a single aldol product?
A)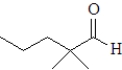
B)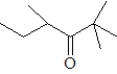
C)
D)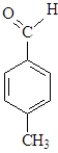
A)

B)

C)

D)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 36 في هذه المجموعة.
فتح الحزمة
k this deck
24
Draw the products of the Claisen condensation of the following substance.Atoms other than carbon and hydrogen are labeled. 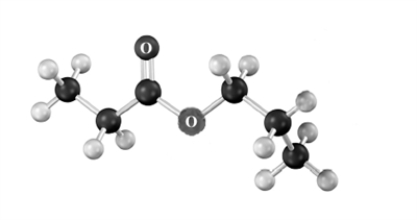


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 36 في هذه المجموعة.
فتح الحزمة
k this deck
25
H3O+
i.
SOCl2,CHCl3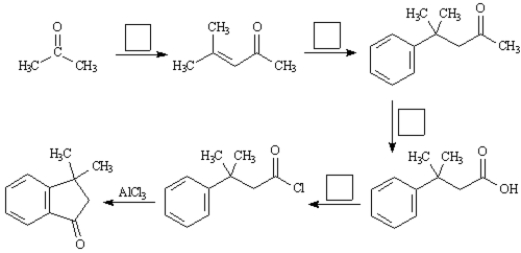
i.
SOCl2,CHCl3


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 36 في هذه المجموعة.
فتح الحزمة
k this deck
26
Which of the following can result in cyclization?
A)aldol condensation
B)Claisen condensation
C)Robinson annulation
D)All can result in cyclization.
A)aldol condensation
B)Claisen condensation
C)Robinson annulation
D)All can result in cyclization.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 36 في هذه المجموعة.
فتح الحزمة
k this deck
27
Which of the following does not characterize carbonyl condensation reactions?
A)rare in biological systems
B)form new carbon-to-carbon bonds
C)enolate ion can act as a nucleophile
D)an α-hydrogen required for an aldol condensation
E)All of these characterize carbonyl condensation reactions.
A)rare in biological systems
B)form new carbon-to-carbon bonds
C)enolate ion can act as a nucleophile
D)an α-hydrogen required for an aldol condensation
E)All of these characterize carbonyl condensation reactions.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 36 في هذه المجموعة.
فتح الحزمة
k this deck
28
Which of the following constitutes a difference between an aldol and a Claisen condensation?
A)basic conditions
B)tetrahedral intermediate
C)enolate ion formation
D)protonation of the intermediate
A)basic conditions
B)tetrahedral intermediate
C)enolate ion formation
D)protonation of the intermediate

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 36 في هذه المجموعة.
فتح الحزمة
k this deck
29
Which of the following would be classified as a Michael donor?
A)propenamide
B)1-nitropentane
C)3-buten-2-one
D)propylpropenoate
A)propenamide
B)1-nitropentane
C)3-buten-2-one
D)propylpropenoate

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 36 في هذه المجموعة.
فتح الحزمة
k this deck
30
Consider the following molecular model.Atoms other than carbon and hydrogen are labeled. 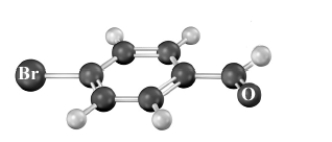 A synthesis is set-up based upon treatment of this compound with NaOH in ethanol.This synthesis aims to produce the following substance.
A synthesis is set-up based upon treatment of this compound with NaOH in ethanol.This synthesis aims to produce the following substance. 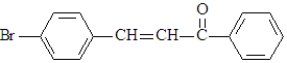 What is wrong with this synthesis?
What is wrong with this synthesis?
 A synthesis is set-up based upon treatment of this compound with NaOH in ethanol.This synthesis aims to produce the following substance.
A synthesis is set-up based upon treatment of this compound with NaOH in ethanol.This synthesis aims to produce the following substance. 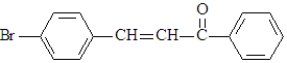 What is wrong with this synthesis?
What is wrong with this synthesis?
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 36 في هذه المجموعة.
فتح الحزمة
k this deck
31
In carbonyl condensation reactions,
A)the carbonyl group acts as an electrophile.
B)the carbonyl group acts as a nucleophile.
C)the carbonyl group acts as both an electrophile and a nucleophile.
D)the carbonyl group acts as neither an electrophile nor a nucleophile.
A)the carbonyl group acts as an electrophile.
B)the carbonyl group acts as a nucleophile.
C)the carbonyl group acts as both an electrophile and a nucleophile.
D)the carbonyl group acts as neither an electrophile nor a nucleophile.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 36 في هذه المجموعة.
فتح الحزمة
k this deck
32
The product of an aldol condensation reaction is generally favored if
A)an aldehyde is disubstituted.
B)a ketone is disubstituted.
C)an aldehyde has no alpha substituents.
D)substituents near the carbonyl are large.
A)an aldehyde is disubstituted.
B)a ketone is disubstituted.
C)an aldehyde has no alpha substituents.
D)substituents near the carbonyl are large.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 36 في هذه المجموعة.
فتح الحزمة
k this deck
33
For which of the following will the aldol reaction go the furthest toward completion?
A)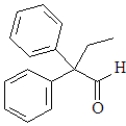
B)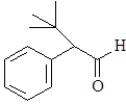
C)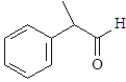
D)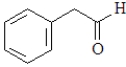
A)

B)

C)

D)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 36 في هذه المجموعة.
فتح الحزمة
k this deck
34
Draw the mechanism of the Claisen condensation reaction between two molecules of methyl acetate in the presence of sodium ethoxide (abbreviated as "EtO-").

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 36 في هذه المجموعة.
فتح الحزمة
k this deck
35
Exhibit 23-11
Give the major organic product(s) for each of the following reactions or reaction sequences.
36.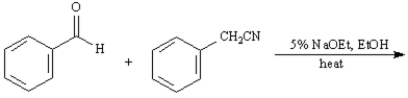 ANSWER:
ANSWER: 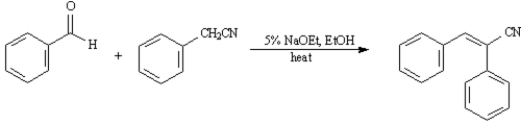
This question was omitted on the printed copy.This placeholder question is here to maintain the numbering system integrity between the printed copy and ExamView.Therefore,it has been marked "do not use on test" in ExamView's question information dialog.As a result,this placeholder question is automatically prevented from being chosen as a test question.
Give the major organic product(s) for each of the following reactions or reaction sequences.
36.
 ANSWER:
ANSWER: 
This question was omitted on the printed copy.This placeholder question is here to maintain the numbering system integrity between the printed copy and ExamView.Therefore,it has been marked "do not use on test" in ExamView's question information dialog.As a result,this placeholder question is automatically prevented from being chosen as a test question.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 36 في هذه المجموعة.
فتح الحزمة
k this deck
36
Draw the mechanism of the Michael reaction between ethyl acetoacetate and hex-5-en-4-one.(The ethyl group is abbreviated as "Et.")

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 36 في هذه المجموعة.
فتح الحزمة
k this deck








