Consider the following table. 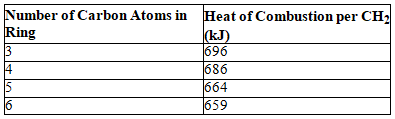
What is the approximate strain energy per CH2 for cyclopropane?
A) 12 kJ
B) 37 kJ
C) 110 kJ
D) 230
Correct Answer:
Verified
Q21: Exhibit 4-4
Label each pair of compounds below
Q22: (-)-Menthol can be isolated from the peppermint
Q23: Which of the following would have the
Q24: In cyclopropane,which of the following strain types
Q25: In general,5-alkyl substituents in 1,3-dioxane exhibit a
Q27: D-Pinitol is an interesting hexahydroxy cyclohexane,whose structure
Q28: In methylcyclohexane:
A)all carbon atoms are sp3 hybridized.
B)ring-carbon
Q29: Consider the two methyl groups indicated with
Q30: Which of the following would produce the
Q31: Consider the following table. ![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents