Using the bond dissociation energies given,calculate DH° for the following reaction. 
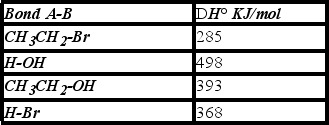
A) +108 KJ/mol
B) -130 KJ/mol
C) -22 KJ/mol
D) +22 KJ/mol
Correct Answer:
Verified
Q14: Which of the following statements is not
Q15: Which of the following statements about elimination
Q16: Which of the Keq corresponds to the
Q17: Which of the following statements about addition
Q18: Which of the Keq corresponds to the
Q20: What kind of reaction does the conversion
Q21: How many transition states and intermediates would
Q22: The DG° (free energy change)for the conversion
Q23: Which reaction is slowest? Q24: Which step would most likely have the![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents