Use the phase digram to answer the following questions: 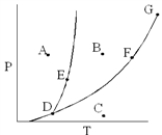
-According to the phase diagram ,which point corresponds to conditions where both liquid and gas phases exist?
A) A
B) B
C) C
D) E
E) F
Correct Answer:
Verified
Q13: Which of the following equations is
Q14: Sulfur dioxide has an enthalpy of vaporization
Q15: Ethanol has a molar heat of vaporization
Q16: At 75.0 °C,water has an equilibrium vapor
Q17: Naphthalene,a substance present in some mothballs,has a
Q19: Water has an equilibrium vapor pressure of
Q20: Use the phase digram to answer the
Q21: Which of the following nonpolar molecules has
Q22: All of the following statements concerning dispersion
Q23: Which of the following substances will exhibit
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents