What process occurs when a substance is at point C on the phase diagram below,and the pressure is decreased (under constant temperature) until the substance is at point E? 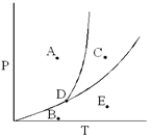
A) condensation
B) vaporization
C) sublimation
D) melting
E) freezing
Correct Answer:
Verified
Q4: Mount Everest rises to a height of
Q5: Carbon tetrachloride,an organic solvent,has a molar heat
Q6: Xenon has a molar heat of vaporization
Q7: All of the following statements are incorrect
Q8: Methyl alcohol,CH3OH,has a vapor pressure of 203
Q10: Freon-113,C2Cl3F3,has an enthalpy of vaporization of 27.04
Q11: The normal boiling point of a liquid
Q12: Sulfur dioxide has a vapor pressure of
Q13: Which of the following equations is
Q14: Sulfur dioxide has an enthalpy of vaporization
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents