What is the balanced chemical equation for the combustion of 4-methyl-1-pentene?
A) 2 C5H10(g) + 15 O2(g) → 10 CO2(g) + 10 H2O( 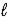 )
)
B) 2 C4H10(g) + 13 O2(g) → 8 CO2(g) + 10 H2O( 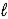 )
)
C) C4H8(g) + 6 O2(g) → 4 CO2(g) + 4 H2O( 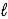 )
)
D) C6H12(g) + 9 O2(g) → 6 CO2(g) + 6 H2O( 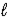 )
)
E) C5H12(g) + 13 O2(g) → 5 CO2 (g) + 6 H2O( 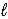 )
)
Correct Answer:
Verified
Q17: Which of the following is the general
Q18: Four of the following compounds are structural
Q19: What is the molecular formula for heptane?
A)
Q20: Which of the following molecules is a
Q21: What is the name of the following
Q23: What is the name of the following
Q24: What type of hydrocarbon is the following
Q25: Which of the following is the expected
Q26: How many structures (i.e.,unique isomers)exist for tribromobenzene?
A)
Q27: Which of the following is the product
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents