Which of the following is not a correct representation of the integrated rate expression for a decomposition first-order reaction?
A) 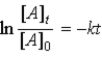
B) 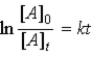
C) 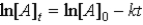
D) 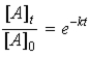
E) 
Correct Answer:
Verified
Q34: At a given temperature,a first-order reaction has
Q35: In general,as temperature increases,the rate of a
Q36: A student analyzed a first-order reaction and
Q37: For the second-order reaction below,the initial concentration
Q38: A second-order reaction starts with an initial
Q40: The decomposition of phosphine,PH3,follows first-order kinetics. 4
Q41: For a chemical reaction,the activation energy for
Q42: The reaction,A + 2B → B2 +
Q43: Consider the following proposed mechanism.If this mechanism
Q44: Calculate the activation energy,Ea,for N2O5(g)→ 2 NO2(g)+
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents