Hydrogen peroxide decomposes into water and oxygen in a first-order process. H2O2(aq) → H2O( 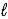 ) + 1/2 O2(g)
) + 1/2 O2(g)
At 20.0 °C,the half-life for the reaction is 3.92 × 104 seconds.If the initial concentration of hydrogen peroxide is 0.52 M,what is the concentration after 7.00 days?
A) 1.2 × 10-5 M
B) 0.034 M
C) 0.074 M
D) 0.22 M
E) 0.52 M
Correct Answer:
Verified
Q20: For a certain reaction of the general
Q21: For the reaction A → B +
Q22: A first-order reaction is 40.0% complete at
Q23: For the first-order decomposition of N2O5 at
Q24: What is the half-life of the first-order
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents