Nickel crystallizes in a face-centered cubic lattice.The density of the nickel is 8.91 g/cm3.What is the volume of a single unit cell?
A) 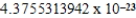 cm3
cm3
B) 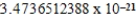 cm3
cm3
C) 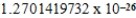 cm3
cm3
D) 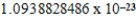 cm3
cm3
E) 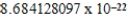 cm3
cm3
Correct Answer:
Verified
Q28: Cesium bromide crystallizes in a primitive cubic
Q29: What is the simplest formula of the
Q30: Rubidium iodide (molar mass 212.4 g/mol)has a
Q31: If an ionic compound with the formula
Q32: Iron(II)sulfide has a primitive cubic unit cell
Q34: Strontium oxide has a face centered cubic
Q35: In what type of unit cell are
Q36: A metal crystallizes in a face-centered cubic
Q37: Lithium chloride crystallizes in a face-centered cubic
Q38: If an ionic compound with the formula
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents