The decomposition of 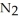
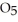 in solution in carbon tetrachloride proceeds via the reaction 2
in solution in carbon tetrachloride proceeds via the reaction 2 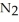
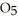 (soln) →
(soln) → 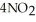 (soln) +
(soln) + 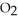 (soln)
(soln)
The reaction is first order and has a rate constant of 4.82 × 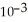
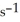 at 64 °C. If the reaction is initiated with 0.073 mol in a 1.00 L vessel, how many moles remain after 151 s?
at 64 °C. If the reaction is initiated with 0.073 mol in a 1.00 L vessel, how many moles remain after 151 s?
A) 0.077 mol
B) 0.053 mol
C) 0.038 mol
D) 15 mol
E) 0.035 mol
Correct Answer:
Verified
Q88: For a reaction that follows the general
Q96: The decomposition of dinitrogen pentoxide is described
Q127: The isomerization of methylisonitrile to acetonitrile
Q128: The rate of disappearance of HBr in
Q129: What is one of the conditions that
Q130: The combustion of ethylene proceeds by the
Q131: Identify a heterogeneous catalyst.
A) CFCs with ozone
B)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents