Silver ions can be precipitated from aqueous solutions by the addition of aqueous chloride: 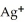 (aq) +
(aq) + 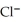 (aq) → AgCl(s) Silver chloride is virtually insoluble in water so that the reaction appears to go to completion. How many grams of solid NaCl must be added to 25.0 mL of 0.376 mol L-1
(aq) → AgCl(s) Silver chloride is virtually insoluble in water so that the reaction appears to go to completion. How many grams of solid NaCl must be added to 25.0 mL of 0.376 mol L-1 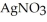 solution to completely precipitate the silver?
solution to completely precipitate the silver?
A) 6.22 × 103 g
B) 0.549 g
C) 0.161 g
D) 9.40 × 10-3 g
E) 1.61 × 10-4 g
Correct Answer:
Verified
Q7: What are the coefficients in front of
Q108: Which of the following compounds is an
Q109: What is the reduction half-reaction for the
Q155: Which pair of compounds is soluble in
Q156: What is the stoichiometric coefficient for oxygen
Q157: Which of the following compounds is an
Q159: H Br , H I , H
Q160: Which of the following solutions will have
Q161: A solution is prepared by adding 1.40
Q162: Lithium and nitrogen react to produce lithium
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents