Automotive air bags inflate when sodium azide decomposes explosively to its constituent elements: 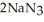 (s) → 2Na(s) +
(s) → 2Na(s) + 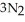 (g) How many grams of sodium azide are required to produce 37.0 g of nitrogen?
(g) How many grams of sodium azide are required to produce 37.0 g of nitrogen?
A) 0.881
B) 129
C) 1.98
D) 85.9
E) 57.2
Correct Answer:
Verified
Q7: What are the coefficients in front of
Q101: Which of the compounds H2C2O4,Ca(OH)2,KOH,and HI behave
Q111: When dissolved in water,KOH behaves as:
A)an acid
Q119: Which one of the following compounds behaves
Q149: What is the oxidation number of the
Q165: What is the oxidation number change for
Q166: Automotive air bags inflate when sodium azide
Q169: How many millilitres of 0.152 mol L-1
Q170: A solution is prepared by mixing 70.0
Q171: Lithium and nitrogen react in a combination
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents