A stock solution of HNO3 is prepared and found to contain 13.7 mol L-1 of 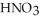 . If 25.0 mL of the stock solution is diluted to a final volume of 0.500 L, what is the concentration of the diluted solution?
. If 25.0 mL of the stock solution is diluted to a final volume of 0.500 L, what is the concentration of the diluted solution?
A) 1.46 mol L-1
B) 274 mol L-1
C) 0.274 mol L-1
D) 685 mol L-1
E) 0.685 mol L-1
Correct Answer:
Verified
Q136: What is the concentration of FeCl3 in
Q148: What is the concentration of NO3- ions
Q150: How many grams of CH3OH must
Q154: If the reaction of phosphate ion with
Q157: A student prepared a stock solution by
Q209: Pure acetic acid (HC2H3O2) is a liquid
Q210: What volume of a 0.540 mol L-1
Q216: How many millilitres of a stock solution
Q217: How many grams of H3PO4 are in
Q218: How many grams of NaOH (MW =
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents