Carbon- 11 decays by positron emission: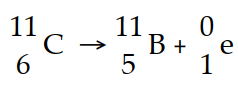
The decay occurs with a release of 2.87 × 1011 J per mole of carbon- 11. When 4.00 g of carbon- 11 undergoes this radioactive decay, _ _ g of mass is converted to energy.
A) 1.28 × 10- 2
B) 1.16 × 10- 3
C) 3.48 × 105
D) 1.16 × 10- 6
E) 8.62 × 102
Correct Answer:
Verified
Q55: A freshly prepared sample of curium- 243
Q56: What happens to the mass number and
Q57: What happens to the mass number and
Q58: What is the rate constant (in min-
Q59: The mass of a proton is 1.00728
Q65: High speed electrons emitted by an unstable
Q142: Conversion of one nucleus into another was
Q145: What happens to the atomic mass number
Q152: Carbon-11,fluorine-18,oxygen-15 and nitrogen-13 are all used in
Q154: _ discovered radioactivity.
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents