The standard reduction potential, E o
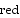
, is proportional to the stoichiometric coefficient.
Correct Answer:
Verified
Q76: The balanced half- reaction in which chlorine
Q77: The balanced half- reaction in which dichromate
Q78: In a voltaic cell, electrons flow from
Q79: The standard cell potential (E°cell) for the
Q80: The relationship between the change in Gibbs
Q83: The electode where reduction occurs is called
Q84: The lithium ion battery has more energy
Q85: Disadvantages of the methanol fuel cell compared
Q102: The standard reduction potential of X is
Q103: In a half reaction,the amount of a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents