A substance has a melting point of 20°C and a heat of fusion of 3.9 × 104 J/kg. The boiling point is 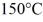 and the heat of vaporization is 7.8 × 104 J/kg at a pressure of 1.0 atm. The specific heats for the solid, liquid, and gaseous phases are 600 J/(kg∙K) , 1000 J/(kg∙K) , and 400 J/(kg∙K) , respectively. The quantity of heat required to raise the temperature of
and the heat of vaporization is 7.8 × 104 J/kg at a pressure of 1.0 atm. The specific heats for the solid, liquid, and gaseous phases are 600 J/(kg∙K) , 1000 J/(kg∙K) , and 400 J/(kg∙K) , respectively. The quantity of heat required to raise the temperature of 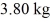 of the substance from
of the substance from 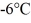 to
to 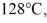 at a pressure of 1.0 atm, is closest to
at a pressure of 1.0 atm, is closest to
A) 620 kJ.
B) 470 kJ.
C) 560 kJ.
D) 210 kJ.
E) 770 kJ.
Correct Answer:
Verified
Q26: How many grams of ice at -13°C
Q30: A 200-g metal container, insulated on the
Q30: A 406.0 kg copper bar is put
Q32: An 80-g aluminum calorimeter contains 380 g
Q33: If you add 700 kJ of heat
Q35: Two metal rods, one silver and the
Q39: A substance has a melting point of
Q40: Two experimental runs are performed to determine
Q41: A solid concrete wall 4.0 m by
Q56: A copper cylinder with a mass of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents