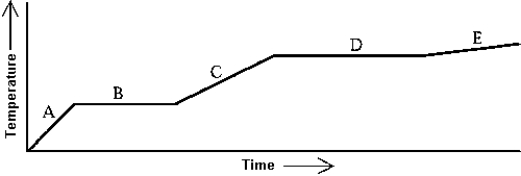
 Heat is added to a substance at a constant rate. The substance starts as a solid and is melted; the liquid is heated and vaporized; finally, the vapor is heated. This process is shown in the graph. Which of the following statements is correct?
Heat is added to a substance at a constant rate. The substance starts as a solid and is melted; the liquid is heated and vaporized; finally, the vapor is heated. This process is shown in the graph. Which of the following statements is correct?
A) The latent heat of fusion is greater than the latent heat of vaporization.
B) The latent heat of vaporization is greater than the latent heat of fusion.
C) The latent heat of vaporization is equal to the latent heat of fusion.
D) The mass of the substance must be known before any statements about the latent heats can be made.
E) The relative sizes of the latent heats depend on the rate at which the heat is added.
Correct Answer:
Verified
Q30: If the heat capacities of both ice
Q109: A 2.0-kg mass of iron specific heat
Q110: The molar specific heat of copper is
Q111: Some water is poured into some ice.
Q112: The temperature of water in the Gulf
Q113: A small lake has a surface area
Q115: If 100 g of steam at 100°C
Q116: You add 50 g of ice cubes
Q117: When a substance changes phase, from solid
Q118: A 2.0-kg mass of iron specific heat
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents