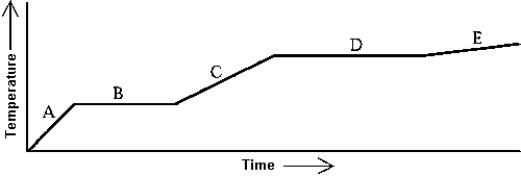
 Heat is added to a substance at a constant rate. The substance starts as a solid and is melted; the liquid is heated and vaporized; finally, the vapor is heated. This process is shown in the graph. The latent heat of fusion can be found by
Heat is added to a substance at a constant rate. The substance starts as a solid and is melted; the liquid is heated and vaporized; finally, the vapor is heated. This process is shown in the graph. The latent heat of fusion can be found by
A) multiplying the length of B in seconds) by the rate at which heat is added, and dividing by the mass of the substance.
B) multiplying the length of D in seconds) by the rate at which heat is added, and dividing by the mass of the substance.
C) multiplying the slope of A by the rate at which heat is added, and dividing by the mass of the substance.
D) multiplying the slope of C by the rate at which heat is added, and dividing by the mass of the substance.
E) multiplying the slope of E by the rate at which heat is added, and dividing by the mass of the substance.
Correct Answer:
Verified
Q28: A group of explorers in the Antarctic
Q101: A 4-kg mass of metal of unknown
Q102: On a hot summer day, water collects
Q103: A 3-kg mass of metal of specific
Q104: A container contains 200 mL of 100%
Q105: If the heat given off by 300
Q107: A container contains 200 mL of 100%
Q109: A 2.0-kg mass of iron specific heat
Q110: The molar specific heat of copper is
Q111: Some water is poured into some ice.
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents