Consider the equilibrium reaction shown below. B2(g) 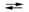 2B(g)
2B(g)
If the rate constants are: kfwd = 7.00 * 10¯5 s¯1 and krev = 2.00 * 10¯5 L mol¯1 s¯1, what is the value of Kc under these conditions?
A) 1.75 * 105
B) 3.50
C) 0.286
D) 5.71 *10¯6
E) 1.40 * 10¯10
Correct Answer:
Verified
Q8: What is the mass-action expression, Qc, for
Q9: What is the mass-action expression, Qc, for
Q10: The reaction quotient for a gas phase
Q11: The reaction quotient, Qc, for a reaction
Q14: Write the mass-action expression, Qc, for the
Q15: (p. Various sections) Which of the following
Q17: What is the mass-action expression, Qp, for
Q18: An equilibrium is established in which both
Q24: The two equilibrium constants for the same
Q32: In order to write the correct mass-action
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents