The heating curve for a substance is shown below.The substance initially is a solid.It then becomes a liquid and a gas.Which of the line segments (I-V) represents the solid-to-liquid phase transition? 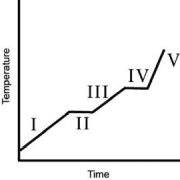
A) I
B) II
C) III
D) IV
E) V
Correct Answer:
Verified
Q44: When one mole of zinc and
Q45: How much energy is needed to
Q46: Which statement below regarding heat capacity
Q47: Which statement below regarding heating curves is
Q48: When trinitrotoluene TNT) detonates according to
Q50: Sodium azide (NaN3) decomposes to form sodium
Q51: Enthalpy change is defined as
A)the energy that
Q52: The reaction of nitrogen with oxygen
Q53: In a steam engine, steam in a
Q54: In a steam engine, steam in a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents