About how long would it take hydrogen cyanide gas (HCN) to travel 10.0 meters at 25.0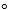 C, when its average kinetic energy is 3.72 kJ/mol?
C, when its average kinetic energy is 3.72 kJ/mol?
(1 J = 1 kg . m2/s2; 1 mol = 6.02 * 1023)
A) 36.3 microseconds
B) 19.1 milliseconds
C) 27.0 milliseconds
D) 275 milliseconds
E) 524 milliseconds
Correct Answer:
Verified
Q19: Which of the following gases will escape
Q20: What is the average kinetic energy
Q21: You need to hold the pressure of
Q22: _-is how gases escape through a hole
Q23: Which set of gases is listed
Q25: The diffusion rate of helium in
Q26: What is the root-mean-square speed of
Q27: Determine the root-mean-square speed of methane (CH4)
Q28: At a given temperature, the effusion rate
Q29: Helium-neon (HeNe) lasers contain a low-pressure mixture
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents