At what point in the following titration curve for a weak acid being titrated with a strong base is the pH equal to the pKa of the acid? The x-axis scale goes from 0.0 mL to 20.0 mL.The sharp rise is at 10.0 mL. 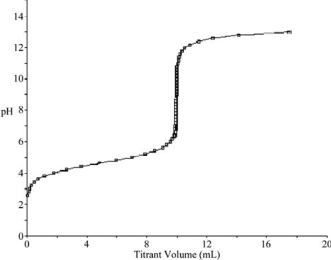
A) 0.0 mL
B) 5.0 mL
C) 9.0 mL
D) 10.0 mL
E) 18.0 mL
Correct Answer:
Verified
Q19: The pKa of a weak acid was
Q20: Which pairs of substances below can be
Q21: At the equivalence point of a strong
Q21: Acid-base indicators change color _
A)exactly when pH
Q22: The following titration curve is most likely
Q23: In which of the following titrations would
Q25: What is true about the pKa of
Q28: What is true about the pKa of
Q29: Phenylephrine (PE; see the structure below) is
Q72: When an acetic acid solution is titrated
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents