A 0.500 g sample of an unknown substance was titrated with a 0.1 M HCl solution.Another 0.500 g sample of it was titrated with a 0.1 M NaOH solution.The resulting titration curves are illustrated here.Given the following possibilities, what is the sample? 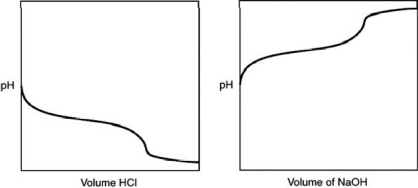
A) Na2CO3
B) NaHCO3
C) H2CO3
D) CO2
E) There is no way to tell.
Correct Answer:
Verified
Q33: A 25.0 mL solution of quinine was
Q50: One brand of extra-strength antacid tablets contains
Q51: Identify the Lewis base in the
Q52: Identify the Lewis acid in the
Q53: A phosphate buffer solution (25.00 mL sample)
Q57: A Lewis base is _
A)an electron-pair acceptor.
B)an
Q57: A solution of the weak acid, HF,
Q59: Identify the Lewis base in the
Q60: A Lewis acid is any species capable
Q60: A 200.0 mL solution of 0.40 M
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents