You are working on a project to recycle nickel and cadmium from old nickel-cadmium batteries that have an iron casing.The batteries are dissolved in aqueous nitric acid, producing a solution containing primarily Ni2+, Cd2+, and Fe3+ cations.One idea is to add sodium hydroxide to neutralize the acid and cause precipitation of Ni(OH) 2, Cd(OH) 2, and Fe(OH) 3.Assume the concentration of each of the cations is 0.100 M before the sodium hydroxide is added.The pH increases as the sodium hydroxide is added.Which compound will precipitate first, and what is the pH at that point? 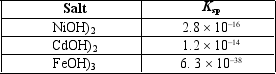
A) Cd(OH) 2; pH = 9.16
B) Fe(OH) 3; pH = 4.81
C) Fe(OH) 3; pH = 1.93
D) Cd(OH) 2; pH= 6.42
E) Ni(OH) 2; pH =7.24
Correct Answer:
Verified
Q84: To simulate the pH of blood, which
Q85: Which of the following compounds does NOT
Q86: Calculate the pH of a solution that
Q87: The Yucca Mountain repository in Nevada, which
Q88: What is the molar solubility of PbF2
Q90: If 500 mL of a solution containing
Q91: The solubility product for Ag2S is written
Q92: Calculate the pH of a solution that
Q94: Stalactites-the long, icicle-like formations that hang from
Q95: Purveyors of salts from the Dead Sea
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents