For each of the numbered bonds in the figure below, identify whether the bond is cis, trans, or neither cis nor trans. 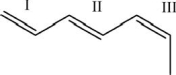
A) I-cis; II-trans; III-cis
B) I-trans; II-cis; III-trans
C) I-neither; II-trans; III-cis
D) I-neither; II-cis; III-trans
E) I-neither; II-cis; II-cis
Correct Answer:
Verified
Q41: Name the compound. Q43: Are the methyl groups in xylene, C6H4(CH3)2, Q44: Consider the 1,2-dichloro derivative of ethylene in Q45: Which of the following statements regarding polymers Q47: Where is the carbon-carbon double bond in Q49: Does this molecule have both cis and Q50: Name the compound.Note: Double and/or triple bonds Q51: How many structural constitutional) isomers are there Q65: Do carbon atoms in aromatic hydrocarbon molecules Q69: Delocalized bonding in aromatic hydrocarbons is possible![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents