Is cyclooctatetraene considered aromatic? The most stable structure at room temperature is shown. 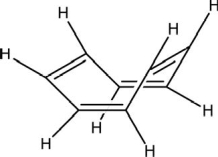
A) Yes, because there are alternating single and double carbon-carbon bonds.
B) Yes, because the electrons in the bonds are delocalized over the ring structure.
C) Yes, there is an even number of electrons involved in bonding.
D) No, the electrons in the bonds are not delocalized over the ring structure.
E) No, the carbons are not sp2 hybridized.
Correct Answer:
Verified
Q47: Where is the carbon-carbon double bond in
Q49: Does this molecule have both cis and
Q50: Name the compound.Note: Double and/or triple bonds
Q51: How many structural constitutional) isomers are there
Q54: Which of the following is NOT a
Q55: Consider the 1,2-dichloro derivative of ethylene in
Q56: A homopolymer is a polymer in which
A)each
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents