Using the bond enthalpy values below, the fuel value for octane is calculated as 44.0 kJ/g, which is close to its experimental value.Predict whether the fuel value of propyl-butylether, C7H16O, will be larger or smaller than that of octane, and calculate an estimate of its fuel value using the data provided.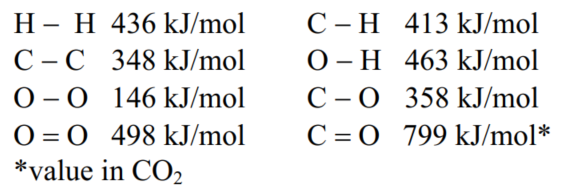
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q127: Write a balanced chemical equation using skeleton
Q128: Suppose monomers of ethylene glycol, HOCH2)2OH, and
Q129: Write the combustion reactions of ethane and
Q129: Note two pros and two cons for
Q130: Identify all the functional groups in the
Q131: Identify all the functional groups in the
Q133: Label the following generic molecules according to
Q134: Identify the following molecules as primary, secondary,
Q135: Draw a few subunits of the addition
Q136: Draw and name all of the isomers
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents