CoF63-absorbs light in the red region of the spectrum, whereas Co(NH3) 63+0 absorbs light on the blue region of the spectrum.This difference means that
A) the splitting of the d orbitals of the two complexes is nearly identical.
B) the value of the crystal field splitting is very small for both complexes.
C) the crystal field splitting is larger for the ammonia complex than for the fluoride complex.
D) the dz2 and 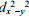 orbitals are higher in energy than the dxy, dxz, and dyz orbitals in CoF63- and just the opposite splitting order for Co(NH3) 63-.
orbitals are higher in energy than the dxy, dxz, and dyz orbitals in CoF63- and just the opposite splitting order for Co(NH3) 63-.
E) the dz2 and dx2-y2 orbitals are lower in energy than the dxy, dxz, and dyz orbitals in CoF63- and just the opposite splitting order for Co(NH3) 63-.
Correct Answer:
Verified
Q60: Complex ions with different ligands have different
Q63: How many unpaired electrons are there in
Q64: Which of the following complexes has the
Q65: Which of the following complexes has the
Q66: As the value of the crystal
Q67: How many unpaired electrons are there in
Q70: Given that the observed absorption maximum
Q71: How many unpaired electrons are there in
Q72: Transition metal ions often absorb visible light
Q73: Which 3d orbitals have a higher energy
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents