Ingestion of mercury, which is poisonous, often is treated using chelation therapy.One agent used in this therapy is dimercaprol, which is shown below.Explain why this compound has a high affinity for mercury atoms or ions and is able to sequester them in a complex. 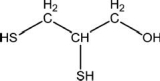
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q97: The complexes shown below are 
Q98: Which of the following statements regarding important
Q99: The complexes shown below are 
Q100: Which of the following regarding crystal field
Q103: Write the equation for the Lewis acid-base
Q104: What is the formula of potassium tetracyanonickelate(II)?
Q105: Describe what is meant by a chelating
Q106: What is the formula of pentaamineiodorhodium(III) iodide?
Q126: Why are compounds of most of the
Q137: Unlike most transition metal compounds, those of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents