The class of the reaction shown in the figure is: 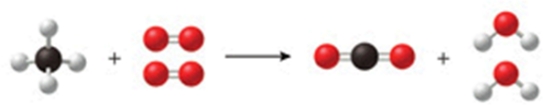
A) double-displacement reaction.
B) decomposition reaction.
C) combustion reaction.
D) combination reaction.
E) single-displacement reaction.
Correct Answer:
Verified
Q44: What is the correct formula for the
Q48: A piece of magnesium metal is placed
Q50: What are the products of the combustion
Q51: The class of the reaction shown in
Q52: What is the product of the combination
Q54: Write and balance the equation for
Q56: A piece of magnesium metal is burned
Q58: Write and balance the equation for
Q59: Write and balance the equation for
Q60: Classify the following reaction: 2C8H18(l) +
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents