At a temperature of 20°C, the volume V (in liters) of 1.33 g of O2 is related to its pressure p (in atmospheres) by the formula 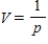 .
.
What is the average rate of change of V with respect to p as p increases from 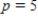 to
to 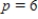 ? What is the rate of change of V with respect to p when
? What is the rate of change of V with respect to p when 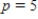 ?
?
Correct Answer:
Verified
Q34: Let Q35: The position of car A and car Q36: Determine all values of x at which Q37: Find the indicated one-sided limit. Q38: Find the values of x for which Q40: Use the graph of the function f Q41: The function that gives the cost of Q42: Refer to the graph of the function Q43: The function P, whose graph follows, gives Q44: Find the one-sided limit, if it exists.![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents