Predict the type of radiation that should be emitted when oxygen-14 undergoes radioactive decay.
A) beta particle ( 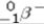 ) emission
) emission
B) positron emission
C) alpha particle emission
D) proton emission
E) neutron emission
Correct Answer:
Verified
Q51: What type of radioactive decay would be
Q52: Iridium-192 has a half-life of 74 days.
Q53: What type of radioactive decay would be
Q54: The half-life of iodine-131 is 8.1 days.
Q55: Predict the type of radiation that should
Q57: The half-life of iodine-123 is 13.3 hours.
Q58: Phosphorus-32 has a half-life of 14 days.
Q59: Select the most stable isotope from the
Q60: The half-life of iodine-131 is 8.1 days.
Q61: Which of the following isotopes would be
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents