The images represent a solution of NaCl, a solution of glucose, and a more dilute solution of glucose. Rank the aqueous solutions in order of highest to lowest freezing point. 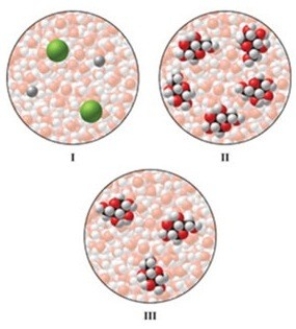
A) I > II > III
B) II > I > III
C) II > III > I
D) III > II > I
E) III > I > II
Correct Answer:
Verified
Q78: When a 25.00 mL sample of NaOH
Q79: The EPA has determined that the maximum
Q80: What volume of 0.1243 M KOH is
Q81: Which of the following aqueous solutions should
Q82: Which of the following properties of water
Q84: Sterling silver is an alloy consisting of
Q85: Vinegar is a mixture of about 5
Q86: The images represent a solution of NaNO3,
Q87: Rank the following solutions in order of
Q88: Which of the following aqueous solutions should
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents