Which is the correct cell diagram for the following electrochemical cell? 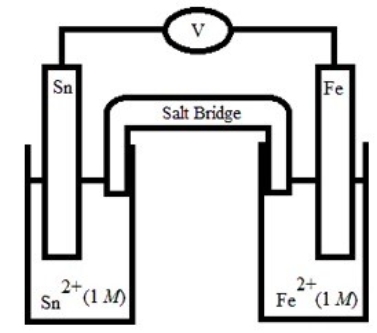
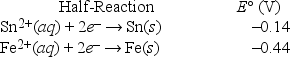
A) Sn(s) | Sn2+(aq, 1.0 M) || Fe2+(aq, 1.0 M) | Fe(s)
B) Sn(s) | Fe2+(aq, 1.0 M) || Sn2+(aq, 1.0 M) | Fe(s)
C) Fe(s) | Sn2+(aq, 1.0 M) || Fe2+(aq, 1.0 M) | Sn(s)
D) Fe(s) | Fe2+(aq, 1.0 M) || Sn2+(aq, 1.0 M) | Sn(s)
E) Sn(s) | Fe(s) || Sn2+(aq, 1.0 M) | Fe2+(aq, 1.0 M)
Correct Answer:
Verified
Q77: What quantity of charge is required to
Q78: Which is the Nernst equation?
A) 
Q79: How much charge must pass through an
Q80: What is the half-reaction that occurs at
Q83: Which of the following is correct?
A) total
Q85: A current of 250. A flows for
Q86: Based on the following electrochemical cell, which
Q87: If the following electrochemical cell is constructed,
Q92: Which of the following elements could be
Q94: Iron objects such as storage tanks and
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents