A friend argues that if mass were really conserved he would never need to refill his gas tank. What explanation do you offer your friend?
A) The atoms (mass) of gasoline are converted into energy by the engine according to E=m 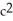 .
.
B) The Law of Conservation of Mass does not apply to reactions involving combustion or explosion of matter.
C) The atoms (mass) of gasoline are converted into exhaust fumes.
D) The oil companies make gasoline in a way that it gets used up so that we are always required to replenish it.
Correct Answer:
Verified
Q4: Steel wool wetted with vinegar is stuffed
Q14: What coefficient is needed in front of
Q15: For the following balanced reaction, which of
Q16: What is wrong with the following depiction
Q17: For the following balanced equation, which has
Q20: For the following balanced reaction, which of
Q22: How many moles of H₂ bonds are
Q24: How much energy, in kilojoules, is released
Q104: Bond energies increase in going from C-N
Q111: ![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents