Set up (but do not solve) an ICE chart for this reaction, given the initial conditions. 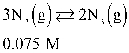
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q80: Explain the importance of the equilibrium constant.
Q81: The _ is the sum of the
Q82: The Ksp is a special type of
Q83: Values of Kb are rarely tabulated. Explain
Q84: What are the three parts of an
Q86: The direction of shift in an equilibrium
Q87: Weak bases have a dissociation constant labeled,
Q88: The relationship of the amounts of reactants
Q89: A(n) _ is a substance that increases
Q90: A(n)_is a change in a condition of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents