As the ideal gas expands from pressure P1 and volume V1 to pressure P2 and volume V2 along the indicated straight line, it is possible that: 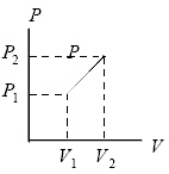
A) the temperature stays constant.
B) the internal energy decreases.
C) the gas is changing state.
D) all of the above are impossible for this particular graph.
Correct Answer:
Verified
Q18: Heat is applied to an ice-water mixture
Q25: A turbine takes in 1 000-K steam
Q38: According to the second law of thermodynamics,which
Q39: An electrical power plant manages to send
Q49: A refrigerator has a coefficient of performance
Q49: According to the first law of thermodynamics,for
Q50: In which system is heat usually transferred
Q51: The PV diagram of a cyclic process
Q56: A Carnot engine runs between a hot
Q57: If one could observe the individual atoms
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents