Figure 2-1
Use the figure below to answer the corresponding question(s) . 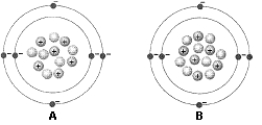
-Figure 2-1 represents:
A) two isotopes of the same element.
B) two different elements.
C) two different ions.
D) an acid and a base.
E) a cation and an anion.
Correct Answer:
Verified
Q11: If atom X contains 14 protons, 13
Q12: In a chemical reaction, the product is:
A)
Q13: An atom has six protons and eight
Q14: Which of the following choices correctly
Q15: An organic compound differs from an inorganic
Q17: Which of the following elements is NOT
Q18: Chlorine has seven electrons in its valence
Q19: When a chemical reaction is at equilibrium:
A)
Q20: Radioisotopes are used in all of the
Q21: The difference between an electrically neutral atom
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents