Solve the problem.
-The pressure of a gas varies jointly as the quantity of the gas (measured in moles) and the temperature and inversely as the volume of the gas. Suppose that the pressure of a particular gas is 1260 kiloPascals (kPa) when
The quantity is 5 moles , the temperature is 280 Kelvin (K) , and the volume is 600 cc. Find the pressure of that
Gas when the number of moles is 7, the temperature is 250 K, and the volume is 420 cc.
A) 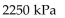
B) 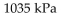
C) 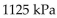
D) 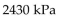
Correct Answer:
Verified
Q240: Solve the problem.
-While traveling at a constant
Q241: Find the quantity indicated.
-z varies directly as
Q242: Solve the problem.
-The power that a resistor
Q243: Solve the problem.
-The voltage across a resistor
Q244: Solve the problem.
-The amount of paint needed
Q245: Solve the problem.
-The time in hours it
Q247: Solve the problem.
-The amount of simple interest
Q248: Solve the problem.
-The volume V of a
Q249: Solve the problem.
-While traveling in a car,
Q250: Find the quantity indicated.
-h varies directly as
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents